b Department of Chemistry, Isfahan University of Technology, Isfahan 84156-83111, Iran
Adrenaline (ADR) is an imperative catecholamine neurotransmitter in the mammalian focal sensory system and natural body liquids. It has been used for the treatment of myocardial infarction, hypertension, bronchial asthma, cardiac arrest, and during cardiac surgery in clinics [1]. Therefore, a simple, fast, and sensitive method is necessary for its determination in both biological fluids and pharmaceutical preparations. Electrochemical detection of ADR at the surface of bare electrodes has a high overpotential, which results in weak electrochemical responses. In addition, the oxidation peak potential of ADR is commonly very close to those of paracetamol (PAC) and tryptophan (Trp), so their signals usually overlap and interfere with each other [2]. Recently, electrochemically conductive polymers have been widely used owing to their excellent unique physical and chemical properties [3, 4]. These electrodes show broad potential windows and can catalyze some electrochemical reactions with high overpotential and poor selectivity [5, 6]. To the best of our knowledge, the electrochemical polymerization of 5-[(2-hydroxynaphthalen-1-yl) diazenyl]isophthalic acid has not been reported in the literature. In this research, we present a facile synthesis method for 5-[(2-hydroxynaphthalen-1-yl) diazenyl] isophthalic acid (NDI). Then, the application of this conductive polymer for simultaneous determination of ADR, PAC, and Trp is studied. 5-[(2-hydroxynaphthalen-1-yl) diazenyl] isophthalic acid (NDI) is an azo compound with two reactive carboxyl groups and one hydroxyl group that can be polymerized on the surface of the GC electrode. The film formation process involves electron transfer from the monomer and polymer to the working electrode by consecutive cyclic voltammetry. The resulting polymerized electrode not only resolves oxidation potential of ADR, PAC, and Trp but also enhances the oxidation current of the three compounds. Incorporation of nanoparticles with conductive polymers can extensively enhance the catalytic activity of the electrode towards many biological molecules. Among nanoparticles, gold nanoparticles are the most stable and receive considerable attention because of their incredible electronic properties and biocompatibility [7, 8]. The constructed electrode has been successfully applied for simultaneous and sensitive determination of ADR, PAC, and Trp in biological samples.
2. Experimental 2.1. Apparatus and reagentsAll electrochemical experiments including cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were performed using a Metrohm instrument, Model 797 VA processor. A conventional three-electrode electrochemical system was used for all electrochemical experiments, which consisted of a working electrode (AuNPs/poly (NDI)/GCE), a platinum wire counter electrode, and Ag/AgCl (3.0 mol L-1 KCl) as a reference electrode. A GCE with a formal surface area of 0.0314 cm2 was used as the basal working electrode. All potentials reported were vs.Ag/AgCl.
All chemicals used were of analytical grade, and doubly distilled water was used throughout. A 1.0 × 10-3 mol L-1 PAC solution was prepared daily by dissolving 0.0150 g PAC (>98%) (from Fluka) in water, and the solution was diluted to 100 mL with water in a 100 mL volumetric flask. The solution was kept in a refrigerator at 4 ℃ in the dark. More dilute solutions were prepared by serial dilution with water. Stock solutions of ADR and Trp (0.0010 mol L-1) were prepared daily by dissolving a suitable amount of ADR and Trp in a small volume of 0.10 mol L-1 H3PO4 solution, and the resulting solution was diluted with water. Phosphate buffer solutions (PBS) with different pH levels were prepared by mixing 0.20 mol L-1 Na2HPO4 and 0.20 mol L-1 NaH2PO4 solutions. The solution pH levels were adjusted by adding 1.0 mol L-1 H3PO4 and/or NaOH solution. Other dilute standard solutions were prepared by appropriate dilution of the stock solutions in the phosphate buffer, pH 3.0.
2.2. Synthesis of 5-[(2-hydroxynaphthalen-1-yl) diazenyl]isophthalic acid5-Aminoisophthalic acid (1.00 g, 5.52 mmol) was dissolved in 12.0 mL of a mixture of DMF and concentrated hydrochloric acid, (2:1, v/v). The solution was then cooled to 5 ℃ and sodium nitrite (0.43 g, 6.2 mmol, 14.2% aq) was added dropwise with vigorous stirring. The resultant mixture was stirred for 30 min, and then 0.79 g (5.52 mmol) of naphthalen-2-ol dissolved in 5 mL of aqueous solution of sodium acetate 5% (w/v) was added portionwise to the diazonium solution. The resulting mixture was stirred for 5 h at 60 ℃. The product was separated upon dilution with cold water and was filtered off and washed several times to reach a neutral state (Scheme 1). The crude dye was purified by re-precipitation of DMF solution in a large amount of water in order to afford a bright yellow powder (91%, vacuum dried at 100 C for 3 h). FT IR (KBr, cm-1): 3470 (m), 3320 (m), 1717 (s), 1660 (s), 1638 (m), 1=1 (s), 1426 (s), 1367 (s), 1264 (s), 1208 (m), 1085 (m), 942 (s), 872 (m), 676 (s). 1H NMR (DMSO-d6): δ 7.41 (m, 2H), 8.01 (m, 2H), 8.31 (d, 2H), 8.71 (d, 1H), 8.84 (d, 2H), 9.02 (s, br, 1H), 13.75 (s, br, 2H). Elemental analysis calculated for C18H12N2O5: C, 64.29%; H 3.06%; N, 8.33%; found: C, 64.01%; H, 2.94%; N, 8.05%.
|
Download:
|
| Scheme 1. Synthesis steps of 5-[(2-hydroxynaphthalen-1-yl) diazenyl]isophthalic acid. | |
2.3. Preparation of AuNPs/poly (NDI) film modified glassy carbon electrode
In order to prepare the surface of the glassy carbon electrode (GCE), we polished it with 0.05 mm alumina slurry on a polishing cloth and rinsed with water. Then, it was sonicated in a mixture of water/ethanol. Subsequently, the electrode was placed in a solution containing 0.10 mol L-1 NaOH containing 1 × 10-3 mol L-1 of NDI, and the cyclic potential sweep was applied in the range of 0.0 and +1.50 at a scan rate of 50 mV s-1 for 30 cycles. After that, the electrode was rinsed and immersed in 0.4 g L-1 HAuCl4 and 0.1 mol L-1 KNO3 to electrodeposit AuNPs for 180 s at -0.2 V [8]. The resulting electrode, AuNPs/poly (NDI)/GCE, was activated by several cyclic voltammetry in the potential range between 0 and +0.80 with a scan rate of 100 mV s-1 in buffer solution (pH 3.0) until a steady state voltammogram was obtained to increase its reproducibility.
2.4. Real sample preparationSerum samples were obtained and stored frozen until the analysis. Proteins from human serum were precipitated by the rapid addition of two volumes of acetonitrile containing 0.1% of trifluoroacetic acid and were immediately mixed by vortexing. Samples were then centrifuged at 5000 rpm for 5 min. The supernatant was filtered using a 0.45 μm pore size filter. For preparation of serum samples, 1.0 mL of each sample was diluted to 10.0 mL in a voltammetric flask by phosphate buffer solution (pH 3.0). Then, 5 mL of this solution was transferred to the voltammetric cell, and it was diluted to 10 mL with phosphate buffer solution. To this solution, different amounts of ADR, PAC, and Trp were added and the recovery percent was obtained by DPV technique and the standard addition method.
Urine samples were centrifuged (2000 rpm for 5 min), and the supernatant was filtered using a 0.45 μm filter. Then, 1.0 mL of the sample solution plus 9.0 mL of 0.10 mol L-1 buffer (pH 3.0) were transferred into the cell to measure ADR, PAC and Trp contents using the standard addition method.
The adrenaline hydrochloride injection solution (specified content of ADR is 1.00 mg mL-1) was analyzed directly after being diluted 50 times with the buffer solution (pH 3.0). An aliquot of 10 mL of this test solution was placed in the electrochemical cell. The potentials were controlled between 0.15 and 1.0 V for cyclic scans at 50 mV s-1. Ipa was measured at the oxidation potential of ADR with standard addition method.
Ten tablets of PAC (labeled 325 mg paracetamol per tablet) were completely ground and homogenized, of which 60 mg was accurately weighed and dissolved with ultrasonication in 25 mL of water. Then, 100 μL of the solution plus 5 mL of the buffer (pH 3.0) was diluted with water in a 10 mL volume flask, and the resulting solution was used for analysis. Then, the diluted sample solutions were placed in an electrochemical cell to determine their concentrations using the DPV method.
3. Results and discussion 3.1. Characterization of AuNPs/poly (NDI) modified electrodeFig. 1 shows the electrode surface morphology of (A) poly (NDI)/ GCE and (B) AuNPs/poly (NDI) film coated GC electrode, characterized by scanning electron microscopy. We can distinctly see the existence of electrodeposited polymer mass and Au nanoparticles on the GCE. The conducting polymer coating increases the surface area, which provides effective support for electrodeposition of AuNPs. Meanwhile, there are a limited number of holes, which help to extend the electrode surface partially. Fig. 1B shows Au nanoparticles are well deposited on the surface of GCE; moreover, it also shows that the surface of the electrode was completely covered with Au nanoparticles. In fact, the polymer chain has been covered by a AuNP layer. This surface presents spheroidal Au particles with well-distributed sizes, and the particles' average diameter is about 60 nm. During the electrodeposition of metal nanoparticles on materials based conducting polymers, the presence of some electron donating functional groups such as nitrogen, oxygen or sulfur in polymer molecules can stabilize nanosized particles by interacting with metal atoms and thus limiting nanoparticle aggregation. Hence, the polymer matrix also acts as the stabilizing medium that prevents agglomeration of metal particles during electropolymerization [9]. As show in the polymerization mechanism, there is only one hydroxyl group as an electron donating which oxidize to a ketone group reversibly, and a deficiency of the aforementioned functional group may cause agglomeration of metal nanoparticles. For instance, Taei et al. investigated deposition of AuNPs on the surface of a conductive polymer film for simultaneous determination of cysteine, uric acid, and tyrosine [10]. The deposited AuNPs have smaller size and agglomeration has not been seen on the surface of the electrode because of an additional hydroxyl group in their conductive polymer structure. In addition, energy-dispersive X-ray spectroscopy (EDX) has also affirmed the formation of the polymer layer and AuNPs on the surface of GCE (Fig. 1C).

|
Download:
|
| Figure 1. Scanning electron microscopy image of (A) poly (NDI) modified glassy carbon electrode, (B) AuNPs/poly (NDI) film modified glassy carbon electrode, and (C) The corresponding EDX spectrum taken from the whole area of (B). | |
3.2. Electrochemical properties of poly (NDI) film
Fig. 2A shows CVs of NDI polymer growth onto a GCE over the range of 0.0 V to +1.50 V at 50 mV s-1 for 30 cycles in a solution containing 0.10 mol L-1 NaOH and 0.0010 mol L-1 5-[(2-hydroxynaphthalen-1-yl) diazenyl] isophthalic acid. The CVs show that the anodic peak current (appearing at +1.30 V due to the oxidation of an OH group) slowly decreased with additional cycles. A decrease in the anodic peak current in the polymerization process with additional cycling may be due to a leaching process in the first steps and self-adjustment of the polymer film thickness at the GCE in further cycles [11]. The effects of various parameters in polymer formation were investigated on the detection of ADR, PAC, and Trp. In fact, the present electrochemical sensor was optimized in terms of the thickness of the polymer film, the potential ranges, and the pH of polymerization. The effect of the film thickness was determined by the number of polymerization scans. The poly (NDI) film was prepared on an activated GCE in 0.001 mol L-1 of monomer and 0.1 mol L-1 NaOH, with the number of cycles ranging from 2 to 35. The Ipa value of analytes increased after increasing the cycle number up to 30 cycles. A further increase in the cycle number caused the current response to be slightly lower. This behavior indicates that the activity of the polymer film is dependent on its thickness, most probably due to a barrier for electron-transfer in thicker films [12]. The potential scan range is also an important factor in the preparation of the poly (NDI) film. If the positive potential value is below +1.5 V or if the lower range is above 0.0 V, no polymerization occurs. Therefore, a potential window from 0.0 to +1.5 V was selected as the electropolymerization range in this paper.

|
Download:
|
| Figure 2. (A) Cyclic voltammograms of the electropolymerization reaction of NDI at the surface of GCE in a solution containing 0.0010 mol L-1 NDI in 0.10 mol L-1 NaOH at 50 mV s-1. (B) Cyclic voltammograms of Au-NPs/poly (NDI)/GCE in PBS (pH 3.0) at various scan rates: (a) 10 mV s-1; (b) 20 mV s-1; (c) 30 mV s-1; (d) 40 mV s-1; (e) 50 mV s-1; (f) 60 mV s-1; (g) 70 mV s-1; (h) 80 mV s-1; and (i) 90 mV s-1. | |
The electropolymerization of NDI varies with the pH of the solution and the electrochemical properties of poly (NDI) film also depends on the film preparation conditions. The optimum pH for the electropolymerization of NDI on the surface of GCE was determined using various electrolytes with different pH values. The pH of the electrolytes was altered from an acidic pH of 4.0 to an alkaline pH of 14.0 using phosphate buffer and NaOH containing 0.001 mol L-1 NDI. The best responses of ADR, PAC, and Trp were found on the surface of poly (NDI) polymerized at pH 13.0. In addition, the NDI has two carboxcylic groups, which facilitate the formation of polymer body as electron withdrawing groups.
Cyclic voltammograms of poly (NDI) modified GC electrode in PBS (pH 3.0) at different scan rates are shown in Fig. 2B. The cyclic voltammograms show a reversible redox couple at EPa=0.29 V and EPc=0.23 V. The plot of IPa versus υ at low scan rates (10-90 mV s-1) exhibits a linear dependence of Ipa on υ and the ratio of IPa to IPc nearly equal to unity. This behavior is consistent with a diffusionless, reversible electron-transfer process [13]. It is also suggested that the reaction of the poly (NDI) film modified electrode is a one-electron transfer process (n=1) because the oxidized form also has aromaticity [14, 15]. Furthermore, the anodic peak current (IPa) is linearly dependent on the scan rate (υ) with the regression equation of I (μA)=15.44 υ (V s-1) + 0.0294 (R2=0.9986).
The influence of the scan rate on the anodic peak current of ADR was studied by cyclic voltammetry. The results showed that the peak current increased by increasing the scan rate. The good linear relationship between υ1/2 and IPa within the scan rate of 10-90 mV s-1 confirmed a diffusion-controlled process on the modified electrode (r2=0.9961). In order to obtain information on the rate-determining step, a Tafel plot was developed for AuNPs/ poly (NDI) modified GCE using the data derived from the rising part of the current-voltage curve at scan rate 20 mV s-1. According to the Tafel equation, the transfer coefficient was calculated as α=0.12.
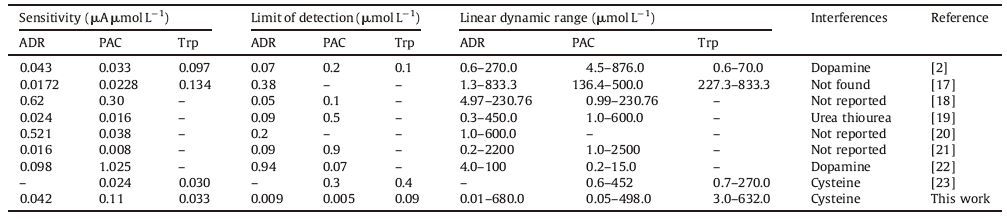
Electrochemical impedance spectroscopy was used to characterize the interface properties of surface AuNPs/poly (NDI) filmmodified GCE during different modification steps. Fig. 3A shows the typical diagrams of [Fe (CN)6]3-/[Fe (CN)6]4- at (a) GCE, (b) poly (NDI)/GCE, (c) AuNPs/poly (NDI)/GCE, and (d) AuNPs/GCE. As shown, the Nyquist plots of the EIS measurement of different electrodeswere recorded in the presence of equivalent 1.0 mmol L-1 [Fe (CN)6]3-/4- and 0.1 mol L-1 KNO3 with 0.15 V polarization potential in frequency: 5.0 × 10 -3 to 105 Hz. The Nyquist plots were fitted with Rs(Q[Rctw]), where Rs refers to the electrolyte resistance, Rct is the charge transfer resistance, Q is the constant phase element, and w is the Warburg impedance coupled to Rct, which is related to Nernstian diffusion. According to the impedance data in Table 1, charge transfer resistance (Rct) of the bare GCE is estimated to be (907Ω). After polymerization with NDI, the Rct decreased (476Ω) suggesting that the conducting polymer enhanced electron transfer. Electrodeposition of AuNPs at the surface of the polymer layer caused further decreases of Rct (293.0Ω) and improved the conductivity of the electrode surface.
|
|
Table 1 Comparison of the electrochemical impedance data in 1.0 mmolL-1 [Fe (CN)6]3-/4- containing 0.1 mol L-1 KNO3. Conditions: polarization potential: 0.15 V, frequency: 5.0× 10-3 to 105 Hz. |
3.3. Electrooxidation of ADR, PAC and Trp at AuNPs/poly (NDI)/GCE
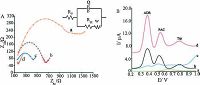
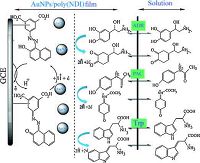
Differential pulse voltammograms of the oxidation of ADR, PAC, and Trp at the surface of (a) bare glassy carbon, (b) poly (NDI)/GCE, (c) AuNPs/GCE, and (d) AuNPs/poly (NDI)/GCE are shown in Fig. 3B. At a bare electrode, the oxidation potential of ADR, PAC, and Trp have the same position +0.68 V. At poly (NDI)/GCE, the oxidation potential of ADR and PAC shifts to less positive potential resulted in three distinguishable sharp peak potentials for each compound. The polymerized electrode separated the oxidation peak potentials for ADR-PAC and PAC-Trp to about 210 mV and 190 mV, respectively. The AuNPs/poly (NDI)/GCE provided significantly higher currents for ADR, PAC, and Trp which proved AuNPs acted as a catalyst to increase the rate of electron transfer (Scheme 2).
|
Download:
|
| Figure 3. (A) Nyquist plots in impedance measurements of electrodes: (a) bare GCE, (b) poly (NDI) modified glassy carbon electrode, (c) AuNPs/poly (NDI)/GCE, and (d) AuNPs modified GCE in 5.0 μmol L-1 K3Fe (CN)6/K4Fe (CN)6 containing 0.10 mol L-1 KNO3. Conditions: polarization potential: 0.15 V, frequency: 5.0 × 10-3 to 105 Hz. (B) Differential pulse voltammograms of 250.0 μmol L-1 ADR, 50.0 μmol L-1 PAC and 100.0 μmol L-1 of Trp at: (a) bare GCE, (b) poly (NDI)/GCE, (c) AuNPs/GCE (d) AuNPs/poly (NDI)/GCE, pH=3.0 with scan rate of 50 mV s-1. | |
|
Download:
|
| Scheme 2. Mechanism of the electropolymerization reaction of NDI at the surface of GCE. | |
3.4. Experimental variables
In order to study the effect of pH on the peak currents and potentials of ADR, PAC, and Trp, differential pulse voltammograms of their solutions were obtained in different pHs. Since all compounds have different pKa, changes in the pH have different effects on the peak currents. In addition, the effect of the pH value of the supporting electrolyte on the electrochemical behavior of the AuNPs/poly (NDI) film coated GC electrode was also investigated. The results show that the anodic peak potential of the polymer was negatively shifted at higher pH values. In addition, the anodic peak current gradually decreased as pH increased from 4.0 to higher values. With an increase in pH, the rate of electrontransfer of the poly (NDI) film was gradually decreased, which is disadvantageous to the electrocatalytic reaction of ADR at the AuNPs/poly (NDI) coated electrode. Therefore, a pH value of 3.0 (PBS, 0.1 mol L-1) was selected for further study. In addition, the relationship of pH vs E0 (peak potential) exhibited a linear dependence for ADR, PAC and Trp. The slope values for all three compounds were close to the slope value of 59 mV/pH. These results confirm that the ratio of the participating protons to the transferred electrons in oxidation process of ADR, PAC and Trp is 1:1.
The DPV parameters, including pulse amplitude, pulse time, and voltage step time changed when the concentrations of ADR, PAC, and Trp on the cell were increased from 20.0 μmol L-1, to 60.0 μmol L-1. The results showed that maximum peak current is obtained with a pulse amplitude of 70.0 mV, a pulse time of 0.06 s, and a voltage step time of 0.6 s. These values were selected for further study.
3.5. Chornoamperometric studiesOne potential step chronoamperometry was applied for the calculation of the diffusion coefficient species of ADR on the AuNPs/poly (NDI) film coated GC electrode by setting the working electrode potential at +0.30 V. The mean value of the diffusion coefficient for ADR was obtained as 1.31 (±0.10) × 10-5 cm2 s-1 according to the Cottrell equation. The rate constant for the chemical reaction between ADR and AuNPs/poly (NDI) film coated GC electrode can be evaluated according to the Galus equation [16]:
| $\frac{{{I}_{\text{C}}}}{{{I}_{\text{L}}}}={{\pi }^{1/2}}{{\gamma }^{1/2}}={{\pi }^{1/2}}{{\left( {{K}_{\text{h}}}{{C}_{\text{b}}}t \right)}^{1/2}}$ | (1) |
where, IC is the catalytic current of AuNPs/poly (NDI) film coated GC electrode in the presence of ADR, IL is the limited current in the absence of ADR, γ=KhCbt (Cb is the bulk concentration of ADR, mol L-1), and Kh and t are the catalytic rate constant ((mol L-1)-1 s-1) and time elapsed (s), respectively. From the slope of the IC/IL vs.t1/2 plot, the value of Kh can be calculated for a given concentration of compound. The catalytic rate constant for the reaction between ADR and poly (NDI) film coated GC electrode was calculated as 2.23 × 103 (mol L-1)-1 s-1. The high Kh value is a product of the sharp feature in the IC/IL vs.t1/2 plot of the catalytic oxidation for ADR at the surface of AuNPs/poly (NDI) film coated GC electrode.
3.6. Stability and reproducibility of the sensorThe stability of the AuNPs/poly (NDI) film coated GC electrode was investigated over a four-week period using 100 μmol L-1 ADR. The differential voltammogram of ADR at the surface of the modified electrode (stored in the laboratory at room temperature) shows that the oxidation peak potential of ADR was retained without any alteration in the peak potential, and the anodic peak current was only decreased by less than 2.1% of the initial oxidation peak current. The reproducibility expressed in terms of the relative standard deviation (RSD) of the same electrode in 10 successive measurements was 0.90%, 0.56%, and 0.71% for ADR, PAC, and Trp, respectively. Furthermore, the RSD values for four electrodes prepared in the same conditions were 1.5%, 2.2%, and 1.6% for ADR, PAC and Trp, respectively, indicating that the proposed method is highly reproducible.
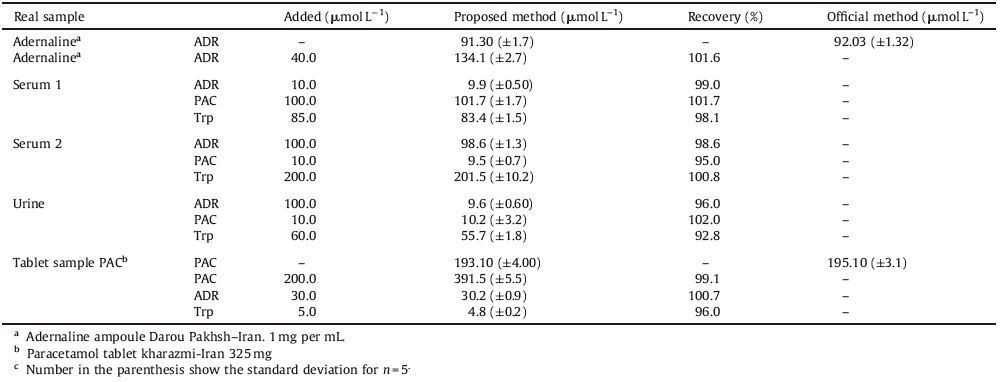
3.7. Simultaneous determination of ADR, PAC and TrpOne of the main objectives of the present study is fabrication of a modified electrode for selective determination of ADR, PAC, and Trp in the presence of each other. Since DPV has a much higher sensitivity and resolution than cyclic voltammetry, DPV was used for simultaneous determination of ADR, PAC, and Trp. In order to investigate sensitivity and selectivity of the fabricated electrode, three experiments were carried out under optimum conditions. In each experiment, DPV was recorded in various concentrations of one of the three compounds, while the concentration of the other two compounds was kept constant. Fig. 4 illustrates the differential pulse voltammograms obtained for the gradual increment of ADR in the presence of 30 μmol L-1 PAC and 150.0 μmol L-1 Trp in 0.1 mol L-1 PBS (pH 3) using AuNPs/poly (NDI)/GCE. This indicates that the determination of ADR has no interference from PAC and Trp. According to the results, no obvious change was observed in the ADR, PAC, or Trp individual oxidation currents while varying the concentration of the other two compounds. It is interesting to note that the sensitivities of the modified electrode towards ADR in the absence and presence of PAC and Trp are virtually the same, which indicates that the oxidation processes of ADR, PAC, and Trp at the AuNPs/poly (NDI) film coated GC electrode are independent and, therefore, simultaneous or independent measurements of the three analytes are possible without any interference. Furthermore, the sensitivity of the modified electrode for determination of ADR, PAC, and Trp in the presence (Fig. 4) and absence of the other two analytes (Fig. 1S in Supporting information) is almost the same. These results indicate that AuNPs/poly (NDI)/GCE can be successfully used for the simultaneous determination of ADR, PAC, and Trp. Furthermore, the detection limit, linear dynamic range, and sensitivity for simultaneous determination of ADR, PAC and Trp using this method were found to be comparable or even better than those of other electrochemical methods reported in the literature (Table 2).

|
Download:
|
| Figure 4. (A) DPV of Au-NPs/poly (NDI) film coated GC electrode in (a) 40.0; (b) 120.0; (c) 190.0; (d) 298.0; (e) 340.0; (f) 480.0; (g)=0.0; (h) 630.0; and (i) 680.0 μmol L-1 ADR, in the presence of 50.0 μmol L-1 PAC and 150.0 μmol L-1 Trp at pH 3.0. (B) DPV graphs of Au-NPs/poly (NDI)/GCE in (a) 1.0; (b) 44.0; (c) 54.0; (d) 98.0; (e) 145.0; (f) 187.0; (g) 224.0; (h) 287.0; (i) 365.0; (j) 433.0; and (k) 479.0 632.0 μmol L-1 PAC in the presence of 200.0 ADR and 50.0 μmol L-1 Trp at pH 3.0. (C) DPV graphs of Au-NPs/poly (NDI)/ GCE in (a) 3.0; (b) 45.0; (c) 80.0; (d) 130.0; (e) 186.0; (f) 245.0; (g) 310.0; and (h) 380.0 μmol L-1 Trp in the presence of 200.0 ADR and 50.0 μmol L-1 PAC at pH 3.0. | |
|
|
Table 2 Comparison of some characteristics of the different modified electrodes for the determination of ADR, PAC and Trp. |
3.8. Interference study
Since the aim of the proposed method is the determination of drug traces in real samples, the effects of various substances as potential interferences on the oxidation current of ADR, PAC and Trp were investigated. The tolerance limit was taken as the maximum concentration of foreign substances which caused no more than±5% relative error in the determination of ADR, PAC, and Trp. The results indicate that no interference was observed for common substances and ions such as Ca2+, Mg2+, nitrate, glucose, fructose, aspartic acid, urea, and starch on the determination of ADR, PAC, and Trp. Although ascorbic acid and cysteine show interference (50-fold of cysteine and 300-fold ascorbic acid), they are not present at significant levels in urine and serum samples. Moreover, if necessary, the interference from ascorbic acid can be minimized by using ascorbic oxidase enzyme, which exhibits high selectivity to the oxidation of ascorbic acid.
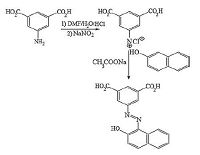
3.9. Analysis of real samplesIn order to evaluate the applicability of the proposed method to the determination of ADR, PAC, and Trp in real samples, the utility of the developedmethod was tested by determining these compounds in biological and pharmaceutical samples by the standard addition method. These solutions were analyzed by DPV, and the concentration of ADR, PAC, and Trp were determined. The results are presented in Table 3. The human urine and serum samples were diluted to a suitable amount with 0.1 mol L-1 PBS pH 3.0 without any treatment. The recoveries of ADR, PAC, and Trp in these samples were in the ranges of 92.8% to 102.0%, indicating that the proposed method could be applied for simultaneous determination of ADR, PAC, and Trp with satisfactory results. In addition, comparisons were made between the results obtained from the proposed method and those from the official method [24-26] to confirm the lack of any significant differences between the two.
|
|
Table 3 Simultaneous determination of ADR, PAC and Trp in real samples and mixtures synthesis samples.c |
4. Conclusions
A highly stable electrochemical sensor based on AuNPs/ poly (NDI) film modified GCE was fabricated for simultaneous determination of ADR, PAC, and Trp. The oxidation potential of ADR and PAC was greatly resolved at a poly (NDI)/GC modified electrode while the bare electrode failed to resolve them. The excellent electrocatalytic activity of the modified electrode was due to the combined properties of the organic polymer and the large specific area of AuNPs. The sensor had a simple construction procedure, high stability, excellent sensitivity, and a wide linear range. The practical application of AuNPs/poly (NDI)/GC electrode was demonstrated by measuring the concentration of ADR, PAC, and Trp in biological samples with satisfactory results. In addition, comparisons were made between the results obtained from the proposed method and those from the official method [24-26] to confirm a lack of any significant differences between the two.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.025.
| [1] | M. Taei, M. Jamshidi, Highly selective determination of ascorbic acid, epinephrine, and uric acid by differential pulse voltammetry using poly (Adizol Black B)-modified glassy carbon electrode. J. Solid State Electrochem. 18 (2014) 673–683. DOI:10.1007/s10008-013-2304-z |
| [2] | M. Taei, M. Shavakhi, H. Hadadzadeh, Simultaneous determination of epinephrine, acetaminophen, tryptophan using Fe2O3(0.5)/SnO2(0.5) nanocomposite sensor. J. Appl. Electrochem. 45 (2015) 185–195. DOI:10.1007/s10800-014-0756-1 |
| [3] | S. Ulubay, Z. Dursun, Cu nanoparticles incorporated polypyrrole modified GCE for sensitive simultaneous determination of dopamine and uric acid. Talanta 80 (2010) 1461–1466. DOI:10.1016/j.talanta.2009.09.054 |
| [4] | A. Zykwinska, W. Domagala, B. Pilawa, M. Lapkowski, Electrochemical overoxidation of poly (3, 4-ethylenedioxythiophene)-PEDOT studied by means of in situ ESR spectroelectrochemistry. Electrochim. Acta 50 (2005) 1625–1633. DOI:10.1016/j.electacta.2004.10.026 |
| [5] | B.D. Malhotra, A. Chaubey, S.P. Singh, Prospects of conducting polymers in biosensors. Anal. Chim. Acta 578 (2006) 59–74. DOI:10.1016/j.aca.2006.04.055 |
| [6] | Ç.C. Koçak, Z. Dursun, Simultaneous determination of ascorbic acid, epinephrine and uric acid at over-oxidized poly (p-aminophenol) film modified electrode. J. Electroanal. Chem. 694 (2013) 94–103. DOI:10.1016/j.jelechem.2013.02.006 |
| [7] | A.A. Ensafi, M. Taei, H.R. Rahmani, T. Khayamian, Sensitive DNA impedance biosensor for detection of cancer, chronic lymphocytic leukemia, based on gold nanoparticles/gold modified electrode. Electrochim. Acta 56 (2011) 8176–8183. DOI:10.1016/j.electacta.2011.05.124 |
| [8] | M. Taei, G. Ramazani, Simultaneous determination of norepinephrine, acetaminophen and tyrosine by differential pulse voltammetry using Au-nanoparticles/poly (2-amino-2-hydroxymethyl-propane-1, 3-dole) film modified glassy carbon electrode. Colloids Surf. B 123 (2014) 23–32. DOI:10.1016/j.colsurfb.2014.09.005 |
| [9] | V.V. Kondratiev, V.V. Malev, S.N. Eliseeva, Composite electrode materials based on conducting polymers loaded with metal nanostructures. Russ. Chem. Rev. 85 (2016) 14–37. DOI:10.1070/RCR4509 |
| [10] | M. Taei, F. Hasanpour, H. Salavati, S.H. Banitaba, F. Kazemi, Simultaneous determination of cysteine, uric acid and tyrosine using Au-nanoparticles/poly (E)-4-(ptolyldiazenyl) benzene-1, 2, 3-triol film modified glassy carbon electrode. Mater Sci. Eng. C 59 (2016) 120–128. DOI:10.1016/j.msec.2015.10.004 |
| [11] | M. Taei, F. Hasanpour, N. Tavakkoli, M. Bahrameian, Electrochemical characterization of poly (fuchsine acid) modified glassy carbon electrode and its application for simultaneous determination of ascorbic acid, epinephrine and uric acid. J. Mol. Liq. 211 (2015) 353–362. DOI:10.1016/j.molliq.2015.07.029 |
| [12] | H.A.A. El-Rahman, A.A. Hathoot, M. El-Bagoury, M. Abdel-Azzem, Electroactive polymer films formed from the Schiff base product of 1, 8-diaminonaphthalene and dehydroacetic acid. I. Preparation and characterization. J. Electrochem. Soc. 147 (2000) 242–247. DOI:10.1149/1.1393182 |
| [13] | H. Yao, Y.Y. Sun, X.H. Lin, Y.H. Tang, L.Y. Huang, Electrochemical characterization of poly (eriochrome black T) modified glassy carbon electrode and its application to simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim. Acta 52 (2007) 6165–6171. DOI:10.1016/j.electacta.2007.04.013 |
| [14] | A.A. Ensafi, M. Taei, T. Khayamian, A. Arabzadeh, Highly selective determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using poly (sulfonazo III) modified glassy carbon electrode. Sens. Actuators B 147 (2010) 213–221. DOI:10.1016/j.snb.2010.02.048 |
| [15] | M. Taei, H. Hadadzadeh, F. Hasanpour, N. Tavakkoli, M. Hadadi Dolatabadi, Simultaneous electrochemical determination of ascorbic acid, epinephrine, and uric acid using a polymer film-modified electrode based on Au nanoparticles/poly (3, 30, 5, 50-tetrabromo-m-cresolsulfonphthalein). Ionics 15 (2015) 3267–3278. |
| [16] | Z. Galus, Fundumentals of Electrochemical Analysis, Ellis Horwood: New York, 1976 . |
| [17] | N. Nasirizadeh, Z. Shekari, H.R. Zare, Electrosynthesis of an imidazole derivative and its application as a bifunctional electrocatalyst for simultaneous determination of ascorbic acid, adrenaline, acetaminophen, and tryptophan at a multi-wall carbon nanotubes modified electrode surface. Biosens. Bioelectron. 41 (2013) 608–614. DOI:10.1016/j.bios.2012.09.028 |
| [18] | B. Devadas, M. Rajkumar, S.M. Chen, Electropolymerization of curcumin on glassy carbon electrode and its electrocatalytic application for the voltammetric determination of epinephrine and p-acetoaminophenol. Colloids Surf. B 116 (2014) 674–680. DOI:10.1016/j.colsurfb.2013.11.002 |
| [19] | T. Tavana, M.A. Khalilzadeh, H. Karimi-Maleh, Sensitive voltammetric determination of epinephrine in the presence of acetaminophen at a novel ionic liquid modified carbon nanotubes paste electrode. J. Mol. Liq. 168 (2012) 69–74. DOI:10.1016/j.molliq.2012.01.009 |
| [20] | M. Mazloum-Ardakani, H. Beitollahi, M.A. Sheikh Mohseni, Simultaneous determination of epinephrine and acetaminophen concentrations using a novel carbon paste electrode prepared with 2, 2'-[1, 2 butanediylbis (nitriloethylidyne)]-bis-hydroquinone and TiO2 nanoparticles. Colloids Surf. B 76 (2010) 82–87. DOI:10.1016/j.colsurfb.2009.10.019 |
| [21] | M. Mazloum-Ardakani, A. Dehghani-Firouzabadi, N. Rajabzade, MCM/ZrO2 nanoparticles modified electrode for simultaneous and selective voltammetric determination of epinephrine and acetaminophen. J. Iran. Chem. Soc. 10 (2013) 1–5. DOI:10.1007/s13738-012-0121-4 |
| [22] | J.B. Raoof, F. Chekin, R. Ojani, Synthesis and characterization of ordered mesoporous carbon as electrocatalyst for simultaneous determination of epinephrine and acetaminophen. J. Solid State Electrochem. 16 (2012) 3753–3760. DOI:10.1007/s10008-012-1807-3 |
| [23] | A.A. Ensafi, H. Karimi-Maleh, S. Mallakpour, Simultaneous determination of ascorbic acid, acetaminophen, and tryptophan by square wave voltammetry using N-(3, 4-dihydroxyphenethyl)-3, 5-dinitrobenzamide-modified carbon nanotubes paste electrode. Electroanalysis 24 (2012) 666–675. DOI:10.1002/elan.v24.3 |
| [24] | A.V. Bulatov, A.V. Petrova, A.B. Vishnikin, A.L. Moskvin, L.N. Moskvin, Stepwise injection spectrophotometric determination of epinephrine. Talanta 96 (2012) 62–67. DOI:10.1016/j.talanta.2012.03.059 |
| [25] | F.A. Mohamed, M.A. AbdAllah, S.M. Shammat, Selective spectrophotometric determination of p-aminophenol and acetaminophen. Talanta 44 (1997) 61–68. DOI:10.1016/S0039-9140(96)02013-9 |
| [26] | S.S.M. Hassan, New spectrophotometric method for simultaneous determination of tryptophan and tyrosine. Anal. Chem. 47 (1975) 1429–1432. DOI:10.1021/ac60358a012 |
 2017, Vol. 28
2017, Vol. 28