Indoles are particular interesting building units owing to their frequent appearance in a vast number of biologically active compounds [1]. After Fischer and Jourdan discovered the wellknown "Fischer indole synthesis" in 1883 [2], numerous synthetic routes to indoles have been reported [3]. Among them, intramolecular cyclization with 2-ethynylaniline derivatives is one of the efficient strategies to assemble indole rings [3, 4]. The presence of transition metals greatly promoted the cyclization reaction of 2-ethynylaniline derivatives [4]. On the other hand, water is the cheapest and environmentally benign solvent. The use of water as solvent in organic synthesis is of great interest [5]. In 2005, Hiroya et al. reported the first example of synthesis of indoles from 2-ethynylanilines via intramolecular cyclization reaction in water catalyzed by a copper salt [4d]. Recently, Song et al. developed a recyclable polystyrene-supported copper catalyst for the cyclization reaction of 2-ethynyl-N-sulfonylanilides in water [4e]. In view of green chemistry, a transition metal-free version of this reaction is more attractive and environment friendly. With the aid of microwave irradiation, Carpita and Ribecai found that the intramolecular cyclization reaction of 2-ethynylanilines could be conducted in water in the absence of any catalysts to give indoles in moderate to good yields [6]. However, the use of microwave irradiation is unfavorable, especially in large-scale synthesis. Therefore, developing more efficient and metal-free approach to indoles is still desirable.
2. Experimental 2.1. General procedure for K2CO3 catalyzed cyclization reaction of 2-ethynylanilines in waterTo a solution of K2CO3 (0.15 equiv., 0.045 mmol) in water (1.5 mL) was added substrate 1 (1 equiv., 0.3 mmol). The resulting mixture was stirred vigorously at 130 ℃ in a sealed tube under an argon atmosphere for 10 h. The reaction solution was cooled to room temperature and extracted by CH2Cl2 (3×5 mL), and the organic phase was collected, dried over anhydrous Na2SO4. Pure product 2 was obtained by direct evaporation under reduced pressure (2a, 2b, 2d, 2e, 2g-2j, 2m, 2n, 2p-2u, 2w or 2x) or by flash chromatography on silica gel (2c, 2f, 2k, 2l, 2o or 2v).
2.2. General procedure for recycling experimentTo a solution of K2CO3 (0.15 equiv., 0.045 mmol) in water (1.5 mL) was added 1j (1 equiv., 0.3 mmol). The resulting mixture was stirred vigorously at 130 ℃ in a sealed tube under an argon atmosphere for 10 h. The reaction solution was cooled to room temperature and extracted by CH2Cl2 (3×5 mL), and the organic phase was collected, dried over anhydrous Na2SO4. Pure product 2j was obtained by flash chromatography on silica gel. The aqueous phase containing the catalyst was reused in the next cycle.
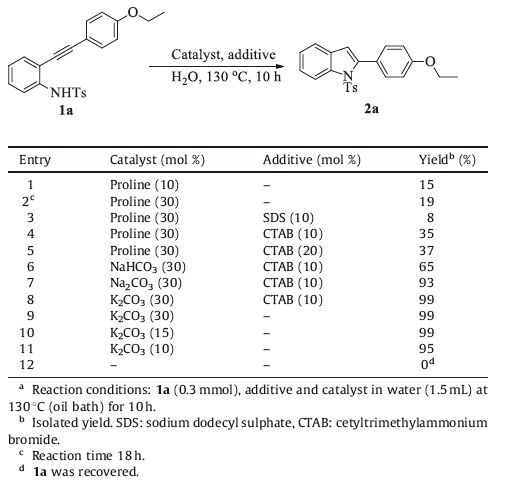
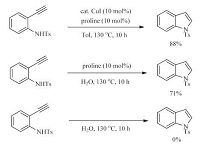
3. Results and discussionDuring our study on domino coupling reaction of 2-ethynylanilines [7], we found that N-tosylated 2-ethynylaniline was cyclized to 1-tosyl-1H-indole in 88% yield in water in the presence of a catalytic amount of CuI and proline (Scheme 1). Interestingly, the intramolecular cyclization reaction occurred even without copper salt by adding 10 mol% proline in water at 130 ℃ giving 1-tosyl-1H-indole in 71% yield (Scheme 1). Unfortunately, when N-tosylated 2-(4-ethoxyphenylethynyl) aniline 1a was used as the substrate, the reaction was rather sluggish and the cyclization product 2a was isolated in poor yield (15%; Table 1, entry 1) even with prolonged reaction time and elevated catalyst loading (19% yield; Table 1, entry 2). It is reported that reaction yield can be enhanced by the addition of surfactants in water [8]. The addition of sodium dodecyl sulphate (SDS) afforded declined yield (entry 3). When cationic surfactant cetyltrimethylammonium bromide (CTAB) was used, the yield was improved to 35% (Table 1, entry 4). Attempt to further enhance the yield of 2a by increasing the amount of CTAB to 20 mol% failed (entry 5). It has been demonstrated that the cyclization reaction of 2-ethynylanilines can be achieved in organic solvents with excesses of strong bases [9], such as EtONa [9a], t-BuOK or KH [9b-e]. More recently, Li et al. found that a catalytic amount of t-BuOK was sufficient to affect the cyclization reaction of 2-ethynylanilines in dry DMSO [10]. Inspired by these reports and the fact that proline can act as a base [11], we envisioned that simple inorganic base might be efficient catalyst for the cyclization reaction of 2-ethynylanilines in water. Indeed, with 30 mol% NaHCO3 as the catalyst, the cyclization product 2a was isolated in 65% yield after 10 h in water (Table 1, entry 6). As expected, the cyclization reaction was further improved when a stronger base Na2CO33 was used as catalyst, and 2a was isolated in excellent yield, 93% (entry 7). With the presence of 30 mol% K2CO3, 1a was cyclised to 2a in almost quantitative yield, 99% (Table 1, entry 8). Notably, the addition of CTAB is not essential (entry 9), and the cyclization reaction is still highly efficient with 15 mol% K2CO3 (Table 1, entry 10). Further lower catalyst loading resulted in inferior yield, 95% (entry 11). No desired product was isolated in the absence of K2CO3 (entry 12).
|
Download:
|
| Scheme 1. Cyclization reaction promoted by proline. | |
|
|
Table 1 Optimization of reaction conditions.a |
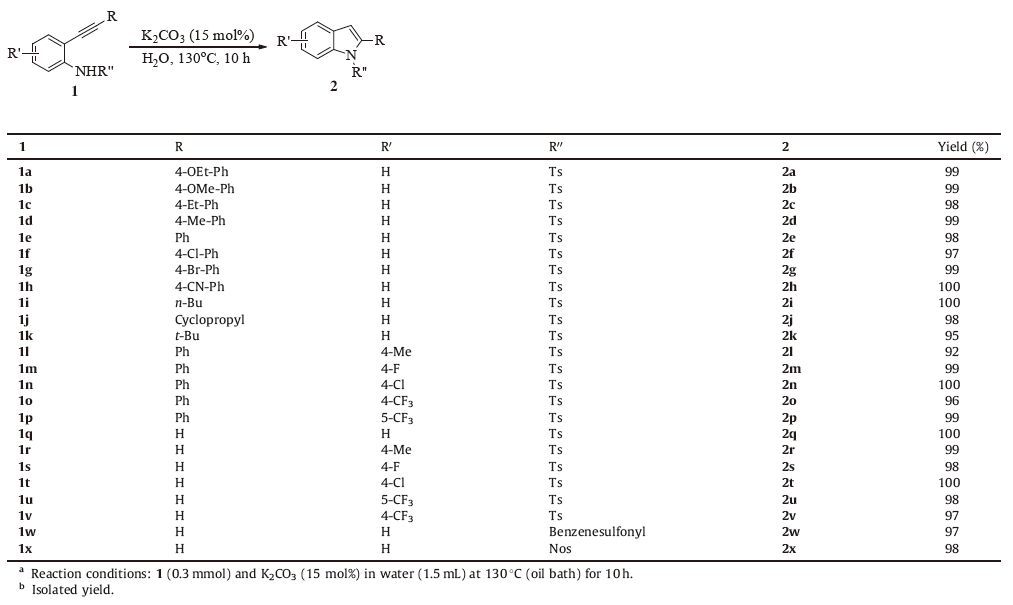
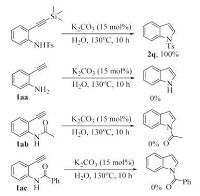
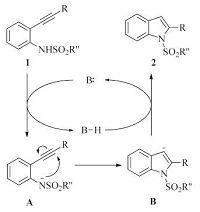
The generality of this method was then tested with various substituted 2-(arylethynyl) anilines, and the results were summarized in Table 2. N-Tosylated 2-(arylethynyl) anilines with electron-donating (1a-1d) or electron-withdrawing (1f-1h) substituents in the aryl ring cyclised to the corresponding 2-arylindoles in high yields. Notably, the nitrile group was found to bewell tolerated under the present reaction condition to afford 2h in quantitative yield. Substrates bearing an alkyl substituent on the alkyne moiety were also applicable (1i-1k). Unlike copper catalyzed cyclization reaction of 2-ethynylanilines in organic solvent [4f], the cyclization reaction still worked highly efficient with a sterically crowded substrate, and 2k was formed in 95% yield under the optimal reaction conditions. 2-Ethynylanilines with different substituent groups on the anilinemoiety were also investigated. 2-Phenylethynyl-N-tosylanilines possessing F, Cl and CF3 substituents at 4 or 5-position reacted smoothly to afford 2m-2p in 96%-100% yields, while relatively lower yield was obtained with substrate bearing electron-donating methyl group (2l, 92%). The intramolecular cyclization proceeded smoothly to give the corresponding indoles (2q-2v) in high yields irrespective of electronic variation on the aniline part of 2-ethynyl-Ntosylanilides. Ethynylanilines having phenylsulfonyl or 4-nitrophenylsulfonyl group on nitrogen were also good substrates furnishing the corresponding cyclization products in high yields (2w and 2x). When 2-(trimethylsilylethynyl) aniline 1y was employed, only the desilylated cyclization product 2q was obtained in quantitative yield (Scheme 2). It is reported that the cyclization reaction is rather sensitive to the acidity of the nitrogen-attached proton [12]. Indeed, no desired product was isolated when 2-ethynylaniline 1aa, N-acetyl aniline 1ab or N-benzoyl derivative 1ac was used as the substrate (Scheme 2). In light of these results and based on the cyclization reaction of 2-ethynylanilines in literature [9, 10], a plausible reaction pathway is shown in Scheme 3. The reaction startedwith the deprotonation of 1 leading to anion intermediate A. Subsequent intramolecular nucleophilic addition gives alkene anion B. Protonolysis delivers the final product 2.
|
|
Table 2 Cyclization reaction of 2-alkynylanilines in water catalyzed by K2CO3.a, b |
|
Download:
|
| Scheme 2. Cyclization reactions with different N-substituted 2-alkynylanilines. | |
|
Download:
|
| Scheme 3. Possible reaction mechanism. | |
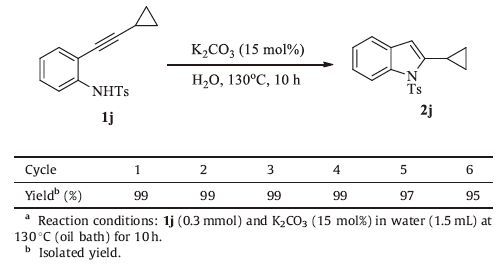
The possibilities of recovery and reusability of the catalytic system were also investigated using 1j by carrying out consecutive cycles. 2j was extracted with CH2Cl2 after each catalytic reaction, and the results are recorded in Table 3. The catalytic system can be recycled at least six timeswith little loss in yield on the 6th run.
|
|
Table 3 Cyclization reaction of 2-alkynylanilines in water catalyzed by K2CO3.a |
4. Conclusion
In conclusion, we have developed a highly efficient method for the synthesis of indoles via K2CO3 catalyzed cyclization reaction of 2-ethynyl-N-sulfonylanilides in water. It is noted that the present procedure offers several advantages, including the absence of transition metal, without the use of excess of strong base, green solvent, the ease of product isolation and cleaner reactions. Moreover, the recovery and reusability of the catalytic system were also investigated, and it could be reused at least six times.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21402137) and Xinmiao Talents Program of Zhejiang Province (No. 2016R430021).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.022.
| [1] | (a) M. Lounasmaa, A. Tolvanen, Simple indole alkaloids and those with a nonrearranged monoterpenoid unit, Nat. Prod. Rep. 17(2000) 175-191; (b) S. Hibino, T. Choshi, Simple indole alkaloids and those with a nonrearranged monoterpenoid unit, Nat. Prod. Rep. 19(2002) 148-180; (c) M. Somei, F. Yamada, Simple indole alkaloids and those with a nonrearranged monoterpenoid unit, Nat. Prod. Rep. 21(2004) 278-311; (d) N.E. Golantsov, A.A. Festa, A.V. Karchava, M.A. Yurovskaya, Marine indole alkaloids containing an 1-(indol-3-yl) ethane-1, 2-diamine fragment, Chem. Heterocycl. Compd. 49(2013) 203-225. |
| [2] | (a) E. Fischer, F. Jourdan, Ueber die hydrazine der brenztraubensa üre, Ber. Deutsch. Chem. Gesell. 16(1883) 2241-2245; (b) E. Fischer, O. Hess, Synthese von indolderivaten, Ber. Deutsch. Chem. Gesell. 17(1884) 559-568. |
| [3] | (a) G.R. Humphrey, J.T. Kuethe, Practical methodologies for the synthesis of indoles, Chem. Rev. 106(2006) 2875-2911; (b) R. Vicente, Recent advances in indole syntheses:new routes for a classic target, Org. Biomol. Chem. 9(2011) 6469-6480; (c) S. Cacchi, G. Fabrizi, Update 1 of:synthesis and functionalization of indoles through palladium-catalyzed reactions, Chem. Rev. 111(2011) PR215-PR283; (d) Y. Oda, N. Matsuyama, K. Hirano, T. Satoh, M. Miura, Dehydrogenative synthesis of C3-azolylindoles via copper-promoted annulative direct coupling of o-alkynylanilines, Synthesis 44(2012) 1515-1520. |
| [4] | (a) L.B. Huang, M. Arndt, K. Gooßen, H. Heydt, L.J. Gooßen, Late transition metalcatalyzed hydroamination and hydroamidation, Chem. Rev. 115(2015) 2596-2697; (b) M. Platon, R. Amardeil, L. Djakovitch, J.C. Hierso, Progress in palladium-based catalytic systems for the sustainable synthesis of annulated heterocycles:a focus on indole backbones, Chem. Soc. Rev. 41(2012) 3929-3968; (c) S. Cacchi, G. Fabrizia, A. Goggiamani, Copper catalysis in the construction of indole and benzo[b]furan rings, Org. Biomol. Chem. 9(2011) 641-652; (d) K. Hiroya, S. Itoh, T. Sakamoto, Mild and efficient cyclization reaction of 2-ethynylaniline derivatives to indoles in aqueous medium, Tetrahedron 61(2005) 10958-10964; (e) S.C. Song, M.N. Huang, W.J. Li, X.H. Zhu, Y.Q. Wan, Efficient synthesis of indoles from 2-alkynylaniline derivatives in water using a recyclable copper catalyst system, Tetrahedron 71(2015) 451-456; (f) K. Hiroya, S. Itoh, T. Sakamoto, Development of an efficient procedure for indole ring synthesis from 2-ethynylaniline derivatives catalyzed by Cu (II) salts and its application to natural product synthesis, J. Org. Chem. 69(2004) 1126-1136. |
| [5] | (a) A. Chanda, V.V. Fokin, Organic synthesis "On Water", Chem. Rev. 109(2009) 725-748; (b) R.N. Butler, A.G. Coyne, Water:nature's reaction enforcer-comparative effects for organic synthesis "In-Water" and "On-Water", Chem. Rev. 110(2010) 6302-6337; (c) M.O. Simon, C.J. Li, Green chemistry oriented organic synthesis in water, Chem. Soc. Rev. 41(2012) 1415-1427. |
| [6] | (a) A. Carpita, A. Ribecai, Microwave-assisted synthesis of indole-derivatives via cycloisomerization of 2-alkynylanilines in water without added catalysts, acids, or bases, Tetrahedron Lett. 50(2009) 6877-6881; (b) A. Carpita, A. Ribecai, P. Stabile, Microwave-assisted synthesis of indole-and azaindole-derivatives in water via cycloisomerization of 2-alkynylanilines and alkynylpyridinamines promoted by amines or catalytic amounts of neutral or basic salts, Tetrahedron 66(2010) 7169-7178. |
| [7] | Z.N. Jin, H.J. Jiang, J.S. Wu, Synthesis of 2-heterocyclic substituted pyrrolidines by copper-catalyzed domino three-component decarboxylative coupling and cyclization reactions. Tetrahedron Lett. 56 (2015) 2720–2723. DOI:10.1016/j.tetlet.2015.04.018 |
| [8] | F. Wang, H. Liu, L.F. Cun, Asymmetric transfer hydrogenation of ketones catalyzed by hydrophobic metal-amido complexes in aqueous micelles and vesicles. J. Org. Chem. 70 (2005) 9424–9429. DOI:10.1021/jo0514826 |
| [9] | (a) J.J. Wang, N. Soundarajan, N. Liu, K. Zimmermann, B.N. Naidu, Highly convergent synthesis of a rebeccamycin analog with benzothioeno (2, 3-a) pyrrolo (3, 4-c) carbazole as the aglycone, Tetrahedron Lett. 46(2005) 907-910; (b) A.L. Rodriguez, C. Koradin, W. Dohle, P. Knochel, Versatile indole synthesis by a 5-endo-dig cyclization mediated by potassium or cesium bases, Angew. Chem. Int. Ed. 39(2000) 2488-2490; (c) C. Koradin, W. Dohle, A.L. Rodriguez, B. Schmid, P. Knochel, Synthesis of polyfunctional indoles and related heterocycles mediated by cesium and potassium bases, Tetrahedron 59(2003) 1571-1587; (d) A.H. Stoll, P. Knochel, Preparation of fully substituted anilines for the synthesis of functionalized indoles, Org. Lett. 10(2008) 113-116; (e) Y. Kondo, S. Kojima, T. Sakamoto, General and facile synthesis of indoles with oxygen-bearing substituents at the benzene moiety, J. Org. Chem. 62(1997) 6507-6511. |
| [10] | D.Y. Li, K.J. Shi, X.F. Mao, Selective cyclization of alkynols and alkynylamines catalyzed by potassium tert-butoxide. Tetrahedron 70 (2014) 7022–7031. DOI:10.1016/j.tet.2014.06.078 |
| [11] | B. List, Proline-catalyzed asymmetric reactions. Tetrahedron 58 (2002) 5573–5590. DOI:10.1016/S0040-4020(02)00516-1 |
| [12] | (a) K. Hiroya, S. Itoh, M. Ozawa, Y. Kanamori, T. Sakamoto, Efficient construction of indole rings from 2-ethynylaniline derivatives catalyzed by copper (II) salts and its application to the tandem cyclization reactions, Tetrahedron Lett. 43(2002) 1277-1280; (b) K.C. Majumdar, S. Samanta, B. Chattopadhyay, A convenient synthesis of pyrrolopyridines and 2-substituted indoles by gold-catalyzed cycloisomerization, Tetrahedron Lett. 49(2008) 7213-7216; (c) A. Yasuhara, Y. Kanamori, M. Kaneko, et al., Convenient synthesis of 2-substituted indoles from 2-ethynylanilines with tetrabutylammonium fluoride, J. Chem. Soc. Perkin Trans. 1(1999) 529-534. |
 2017, Vol. 28
2017, Vol. 28