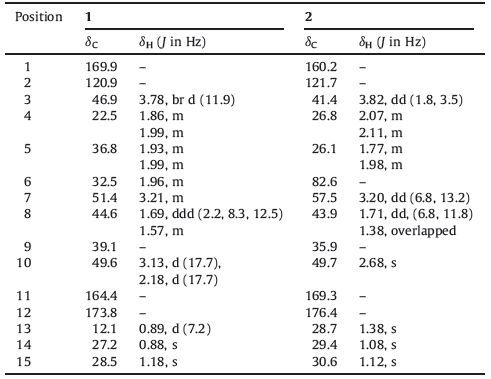
As the second largest ecological group of marine fungi, various chemical entities with unique structures and potent bioactivities which have high potential for exploitation in agriculture, medicine and industrial uses have been isolated from mangrove fungi [1, 2]. They are also suggested as promising sources for screening new natural products [2]. The tremulanes constitute a class of unusual sesquiterpenes that were first isolated from the aspen tree rotting fungus Phellinus tremulae in 1993 [3]. Thus far, more than 50 compounds of this class have been reported [4-9]. However, tremulane sesquiterpene lactones were rarely reported, and none of them have been isolated from mangrove endophytic fungi. In this paper, we report the isolation and structure elucidation of two new tremulane sesquiterpene lactones coriolopsin A (1) and coriolopsin B (2) , together with two known tremulane sesquiterpenes conocenol C (3) and ceriponol E (4) from a mangrove endophytic fungus, Coriolopsis sp. J5 (Fig. 1) . Herein the isolation, structure elucidation were reported.
|
Download:
|
| Figure 1. Structures of compounds 1-4. | |
2. Experimental 2.1. General
The NMR spectra were recorded on an AV-500 spectrometer (500 MHz for 1H NMR and 125 MHz for 13C NMR; Bruker), using the solvent residue signal as the internal standard. Optical rotations were recorded using a Rudolph Autopol III polarimeter (Rudolph Research Analytical, Hackettstown, USA). The UV spectra were obtained from a DU-800 spectrometer (Beckman, Brea, USA). The IR spectra (KBr pellets) were run on a 380 FT-IR instrument from Nicolet (Thermo, Pittsburgh, USA). Column chromatography was performed with ODS gel (20-45 mm, Fuji Silysia Chemical Co., Ltd., Durham, USA), Sephadex LH-20 (Merck, Darmstadt, Germany) and silica gel (60-80, 200-300 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). TLC was carried out on silica gel G precoated plates (Qingdao Haiyang Chemical Co., Ltd.), and spots were detected by spraying with 5% H2SO4 in EtOH followed by heating.
2.2. Fungal materialThe mangrove endophytic fungus Coriolopsis sp. J5 was isolated from healthy branches of Ceriops tagal, collected from Dong Zhai Gang Mangrove National Nature Reserve in Hainan Province, China, in 2011. The fungus was identified by sequence analysis of the ITS region of its 18s rDNA. A BLAST search result indicated that the sequence was most similar (99%) to the sequence of Coriolopsis sp. (compared to AY336771.1) . The voucher sample was stored at the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, and maintained on potato dextrose agar (PDA) slant at 4 ℃.
2.3. Fermentation, extraction, and isolationThe fungus stored on PDA medium was inoculated and cultured on PDA agar for 7 days. Seed medium (potato 200 g, dextrose 20 g, distilled water 1000 mL) in 500 mL × 10 Erlenmeyer flasks was inoculated with fungus and incubated at room temperature for 4 days on a rotating shaker (120 rpm). Production medium of solid rice in 1000 mL flasks (rice 50 g, distilled water 200 mL) was inoculated with seed solution (10 mL) one for one. Flasks were incubated at room temperature under static conditions and daylight for 60 days, cultures from 200 flasks were harvested for the isolation of substances. Following incubation, the mycelia and solid rice medium were extracted with EtOH through cheesecloth. Subsequently, the filtrate was extracted three times with an equal volume of Petroleum ether, EtOAc and n-BuOH, successively.
The EtOAc fraction (129.0 g) was chromatographed on a silica gel column using a step gradient elution of CHCl3-MeOH (1:0-0:1, v/v) to afford ten fractions (Fr. 1-Fr. 10) . Fr. 5 (9.9 g) was applied to octadecyl silane (ODS) gel with gradient elution of MeOH-H2O (3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1, 1:0) to yield 19 subfractions (Fr. 5-1-Fr. 5-19) . Subfraction Fr. 5-7 (354.2 mg) was chromatographed on a Sephadex LH-20 column using CHCl3/MeOH (1:1) and then chromatographed on silica gel column with gradient elution of Petroleum ether-EtOAc (20:1, 18:1, 15:1, 11:1) to give compound 3 (4.8 mg). Subfraction Fr. 5-8 (411.0 mg) was chromatographed on silica gel column with gradient elution of Petroleum ether- EtOAc (15:1, 11:1, 10:1) and then chromatographed on a Sephadex LH-20 column using acetone to give compound 2 (6.8 mg). Fr. 7 (20.0 g) was applied to octadecyl silane (ODS) gel with gradient elution of MeOH-H2O (3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1, 1:0) to yield 12 subfractions (Fr. 7-1-Fr. 7-12) . Subfraction Fr. 7-2 (255.8 mg) was chromatographed on silica gel column with gradient elution of Petroleum ether-EtOAc (5:1) and then chromatographed on a Sephadex LH-20 column using acetone to give compound 4 (8.8 mg). Subfraction Fr. 7-9 (346.5 mg) was chromatographed on silica gel column with gradient elution of Petroleum ether-EtOAc (15:1) and then chromatographed on a Sephadex LH-20 column using acetone to give compound 1 (21.3 mg).
Coriolopsin A (1) Colorless needle crystal; m.p. 192.1-193.7 ℃; [α]D25 +87.6 (c 0.05, MeOH); UV (MeOH) λmax (log e): 239 (3.74) nm; IR (KBr) νmax (cm-1) : 2923, 1645 (C55O), 1378, 1037, 1039; For 1H NMR and 13C NMR spectral data, see Table 1; HR-ESIMS m/z: [M+H]+ 249.1483 (calcd. for 249.1485) .
|
|
Table 1 1H NMR (500 MHz) and 13C NMR (125MHz) spectroscopic data for 1 and 2 (1 in acetone-d6, 2 in CD3OD). |
Coriolopsin B (2) Colorless oil; [a]D 25 -21.8 (c 0.05, MeOH); UV (MeOH) lmax (log e): 240 (3.21) nm; IR (KBr) nmax (cm-1) : 3402, 2925, 1632 (C55O), 1384, 1053; For 1H and 13C NMR spectral data, see Table 1; HR-ESIMS m/z: [M+Na]+ 287.1253 (calcd. for 287.1254) .
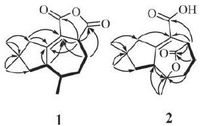
Crystal data of coriolopsin A (1) : Colorless crystals of 1 were obtained in acetone-MeOH (10: 1, v/v). The data collection was acquired on a Bruker D8 QUEST diffractometer (Bruker). All intensity measurements were performed using graphite monochromated Cu Ka radiation (l = 0.71073Å ). In monoclinic, space group P21/n, unit cell dimensions a = 8.4525 (14) Å , b = 6.2926 (10) Å , c = 12.938 (2) Å , α = 90°, β = 103.044 (7) 8, γ = 90°, V = 670.39 (19) Å , Z = 2, Dx = 1.230 g/cm-3, F(0 0 0) = 268.0, m = 0.678 mm-1, the final R = 0.0629 and wR = 0.1464 for 2351 observed reflections [I > 2σ (I)] (see Fig. 3) . Crystallographic information file of Compound 1 with Cambridge Crystallographic Data Centre (CCDC) reference number 1459000 has been deposited at the CCDC, and can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/deposit/.
|
Download:
|
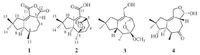
| Figure 2. Key 1H-1H COSY and HMBC correlations of compound 1 and 2. | |

|
Download:
|
| Figure 3. X-ray crystallographic structure of compound 1. | |
2.4. Biological assays
Cytotoxic activity was tested by the MTT method as described previously [10]. Three cancer cell lines, Human chronic myelogenous leukemia cell line (K562) , human gastric carcinoma cell line (SGC-7901) , and human hepatocellular carcinoma (BEL-7402) were used. Antibacterial activity was determined by the filter paper disc diffusion method against Staphylococcus aureus (ATCC51650) , Ralstonia solanacearum, Fusarium oxysporum f.sp. cubense race 4, Fusarium oxysporum f.sp. niveum, Fusarium oxysporum f.sp.vasinfectum and Candida albicans (ATCC 10231) [11].
3. Results and discussionCompound 1 was obtained as colorless needle crystal. The molecular formula of 1 was deduced as C15H20O3 by HR-ESIMS at m/z 249.1483 [M+H]+ (calcd. for C15H20O3, 249.1485) and 13C NMR spectral data, corresponding to six degrees of unsaturation. This was corroborated by the 13C NMR and DEPT NMR spectra, which displayed 15 resonances for one sp3 quaternary carbon (δC 39.1) , two disubstituted olefinic carbons (δC 169.9 and 120.9) , three methines (δC 51.4, 46.9 and 32.5) , four methylenes (δC 49.6, 44.6, 36.8 and 22.5) , three methyls (δC 28.5, 27.2 and 12.1) , and two ester carbonyls (δC 173.8 and 164.4) . These observations, in combination with the molecular formula, revealed that 1 possessed three rings. The above-mentioned data exhibited similarities with those of Tremulenediol A [3], except that two ester carbonyls signals of 1 replace the corresponding two hydroxymethyl signals of Tremulenediol A. 1H-1H COSY correlations of H-3/H-4/H-5/H-6/H-7/H-8 and H-6/H-13, and HMBC correlations from H3-14 (δH 0.88) and H3-15 (δH 1.18) to C-8 (δC 44.6) , C-10 (δC 49.6) and C-9 (δC 39.1) , from H2-10 (δH 3.13 and 2.18) , H1-7 (δH 3.21) and H1-3 (δH 3.78) to C-1 (δC 169.9) and C-2 (δC 120.9) , from H2-4 (δH 1.99 and 1.86) to C- 2 (δC 120.9) , corroborated the existence of the C-1-C-10 substructure and the positions of CH3-13 (δC/H 12.1/0.89) , CH3- 14 (δC/H 27.2/0.88) , and CH3-15 (δC/H 28.5/1.18) . The presence of the dihydrofuran-2, 5-dione moiety was deduced by the molecular formula in combination with the key HMBC correlations from H-3 (δH 3.78) to C-11 (δC 164.4) and C-12 (δC 173.8) (see Fig. 2) . Finally, [M+Na]+ 1 was determined by single crystal X-ray diffraction analysis (see Fig. 3) . The final refinement on the Cu Ka data resulted in a Flack parameter of -0.03 (11) , allowing an unambiguous assignment of the complete absolute configurations of all the chiral centers as 3S, 6R, and 7R.
Compound 2, obtained as colorless oil and was assigned a molecular formula of C15H20O4, as determined by HR-ESIMS (found [M+Na]+ 287.1253, calcd. for 287.1254) and nuclear magnetic resonance (NMR) data, corresponding to six degrees of unsaturation. This was corroborated by the 13C NMR and DEPT NMR spectra, which displayed 15 resonances for one ester carbonyl (δC 176.4) , one carboxylic carbonyl carbon (δC 169.3) , two disubstituted olefinic carbons (δC 121.7 and 160.2) , two sp3 quaternary carbons (δC 35.9 and 82.6) (where one was oxygenated), two allylic sp3 methines (δC 57.5 and 41.4) , four endo-cyclic methylenes (δC 49.7, 43.9, 26.8 and 26.1) , and three methyls (δC 30.6, 29.4 and 28.7) . These observations, in combination with the molecular formula, revealed that 2 possessed three rings. Comparison of NMR data between 1 and 2 suggested that their structures were closely related. The main difference was that the CH-6 in 1 was replaced by an oxygenated quaternary carbon in 2. These data, together with that an additional O atom was present in the molecular formula of 2 compared with that of 1, indicated that the dihydrofuran-2, 5-dione moiety in 2 was hydrolyzed and then the -COOH at C-3 (δC/H 41.4/3.82) was linked with C-6 (δC 82.6) to afford an ester bridge. HMBC and 1H-1H COSY data as shown in Fig. 2 supported this assignment. In the ROESY spectrum, correlations of H-7 (δH 3.20) with H3-14 (δH 1.08) and H3-13 (δH 1.38) , were observed, suggesting that these protons were on the same face, and consequently the ester bridge was on the other face (see Fig. 4) .

|
Download:
|
| Figure 4. Key ROESY correlations of compound 2. | |
The structures of compounds 3-4, which have been isolated from Coriolopsis sp. J5, were identified by comparison of their spectroscopic data with those reported in the literatures as conocenol C (3) [4] and ceriponol E (4) [7]. All compounds were isolated from the fermentation extract of endophytic fungus Coriolopsis sp. J5 from Ceriops tagal for the first time.
All compounds were evaluated for cytotoxic activities against three human tumour cell lines (K562, SGC-7901, and BEL-7402) and antibacterial activities against Staphylococcus aureus (ATCC51650) , Ralstonia solanacearum, Fusarium oxysporum f. sp. cubense race 4, Fusarium oxysporum f.sp. niveum, Fusarium oxysporum f.sp. vasinfectum and Candida albicans (ATCC10231) . However, none of the compounds showed obvious cytotoxic or antibacterial activities.
4. ConclusionTwo new tremulane sesquiterpenes coriolopsin A (1) and coriolopsin B (2), along with two known ones conocenol C (3) and ceriponol E (4), were isolated from the fermentation extract of endophytic fungus Coriolopsis sp. J5 from Ceriops tagal. The structures of 1-4 were elucidated by NMR experiments with the aid of X-ray diffraction analysis. In the bioassay, none of the compounds showed obvious cytotoxic or antibacterial activities.
AcknowledgmentsThis research was financially supported by National Natural Science Foundation of China (Nos. 41406083, 41506096) and National Nonprofit Institute Research Grant of CATAS-ITBB (No.ITBB2015ZY04, ITBB2015RC02) .
| [1] | K. Nithya, J. Muthumary, Bioactive metabolite produced by Phomopsis sp., an endophytic fungus in Allamanda cathartica Linn. Rec. Res. Sci. Technol. 3 (2011) 44–48. |
| [2] | J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep, Marine natural products. Nat. Prod. Rep. 27 (2010) 165–237. DOI:10.1039/b906091j |
| [3] | W.A. Ayer, E.R. Cruz, The tremulanes, a new group of sesquiterpenes from the aspen rotting fungus Phellinus tremulae. J. Org. Chem. 58 (1993) 7529–7534. DOI:10.1021/jo00078a035 |
| [4] | D.Z. Liu, F. Wang, J.K. Liu, Sesquiterpenes from cultures of the basidiomycete Conocybe siliginea. J. Nat. Prod. 70 (2007) 1503–1506. DOI:10.1021/np070140n |
| [5] | Z.Y. Zhou, J.G. Tang, F. Wang, Z.J. Dong, J.K. Liu, Sesquiterpenes and aliphatic diketones from cultures of the basidiomycete Conocybe siliginea. J. Nat. Prod. 71 (2008) 1423–1426. DOI:10.1021/np8002657 |
| [6] | X.L. Wu, S. Lin, C.G. Zhu, Homo- and heptanor-sterols and tremulane sesquiterpenes from cultures of Phellinus igniarius. J. Nat. Prod. 73 (2010) 1294–1300. DOI:10.1021/np100216k |
| [7] | Y.M. Ying, W.G. Shan, L.W. Zhang, Z.J. Zhan, Ceriponols A-K, tremulane sesquitepenes from Ceriporia lacerate HS-ZJUT-C13A, a fungal endophyte of Huperzia serrata. Phytochemistry 95 (2013) 360–367. DOI:10.1016/j.phytochem.2013.07.025 |
| [8] | J. Meng, Y.Y. Li, Y.X. Ou, New sesquiterpenes from Marasmius cladophyllus. Mycology 2 (2011) 30–36. DOI:10.1080/21501203.2011.554908 |
| [9] | E.R. Cruz, The biosynthesis of the new tremulane sesquiterpenes isolated from Phellinus tremulae. Can. J. Chem. 75 (1997) 834–839. DOI:10.1139/v97-101 |
| [10] | Y.B. Zeng, H.G. Gu, W.J. Zuo, Two new sesquiterpenoids from endophytic fungus J3 isolated from mangrove plant Ceriops tagal. Arch. Pharm. Res. 38 (2015) 673–676. DOI:10.1007/s12272-014-0448-8 |
| [11] | S.Y. Xu, R.L. Bian, X. Chen, Methods of Pharmacology Experiment, People's Sanitation Press, Beijing, China, 2003, pp. 1651-1653. |
 2017, Vol. 28
2017, Vol. 28