QDs, known as colloidal semiconductor nanoparticles, have aroused more and more attention in the past few decades on account of their unique and outstanding optical and electronic properties [1-3]. Compared with conventional dyes, QDs have a lot of advantages such as large Stokes shifts, large surface-to-volume ratios, broad excitation spectra, good photo stability and sizedependent emission-wavelength tenability [4, 5]. Owing to their excellent superiority, QDs such as C, N, Si and metal-based quantum dots [6-9] have been extensively utilized in the fields of multicolored photoluminescent probes [10] and biological luminescent labels [11], as well as display devices [12].
CL is defined as the emissive light through chemical reactions [13, 14]. Specific excited species are formed and subsequently deactivated to the ground state along with light emission. Since it has low background scattering light interference and shows excellent sensitivity, rapidity as well as safety, CL has been widely employed in analytical area [15-17]. In recent years, CL has been applied to numerous fields such as chip technique [18], environmental monitoring [19], drug screening [20], clinical diagnosis [21] and food safety [22].
Contemporarily, various QDs have been synthesized and broadly employed in CL analysis. Considerable researches have revealed the application of QDs in the CL reactions and corresponding results indicated that several classic CL systems had been established to achieve enhanced CL performance [23-28]. The CL behavior of carbon dots was investigated in the presence of acidic KMnO4 or cerium (IV). The introduction of either KMnO4 or cerium (IV) into carbon dots resulted in CL with high intensity, and the CL intensity was dependent on the concentration of carbon dots in a certain range [29]. Graphitic carbon nitride quantum dots (g-CNQDs) were prepared and strong CL emission was observed when NaClO solution was injected into g-CNQDs while no CL emission was obtained for the control solution [30]. Electrogenerated CL from silicon nanocrystal QDs was studied, and relevant result showed a significant red shift from the PL maximum of the same silicon nanocrystal QDs [31]. Besides, metal-based QDs were also widely used for CL analysis. For example, different types of Mn-doped ZnS QDs were fabricated and applied to the study of the CL reaction of hydrogen peroxide and periodate. The results supported the thought that the size of QDs, stabilizing agents and silica film protection had a great effect on the CL reaction [32]. However, QDs-based CL analyses were confined to some ordinary systems, and it hindered the development of original CL emitting species. Establishment of novel CL systems was urgently demanded, which extremely contributed to determing the concentration of specific molecules and investigating the property of QDs conversely.
In the current research, CL properties of CdTe/CdS/ZnS QDs were firstly discovered when mixed with KIO4 solution directly. The CL intensity was significantly impacted by the concentration and pH. With the aid of ESR, the effects of radical scavengers and flow injection CL spectra analysis, mechanism of CL in KIO4-CdTe/ CdS/ZnS QDs system could be speculated. This investigation opened new sight into the optical characteristics of CdTe/CdS/ZnS QDs and expanded QDs-based CL system.
2. Experimental 2.1. Chemical reagentsAnalytical grade chemicals were used during the experiments. CdTe/CdS/ZnS QDs were obtained from Beijing Beida Jubang Science & Technology Co., Ltd. (Beijing, China) and KIO4 from Beijing Zhonglian Chemical Reagents (Beijing, China). Whereas, NaN3, thiourea, ascorbic acid, H2SO4 and NaOH were purchased from Beijing Chemical Reagent Co., (Beijing, China). Nitro blue tetrazolium chloride (NBT) was obtained from Nacalai Tesque Inc. (Tokyo, Japan) and DMPO (5, 5-dimethyl-1-pyrroline N-oxide) from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan). 2, 2, 6, 6-tetramethyl-4-piperidine (TEMP) was bought from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were used as received without further purification, and deionized water was used in all experiments.
2.2. ApparatusThe CL was carried out with a BPCL luminescence analyzer (Department of Chemistry, Tsinghua University, Beijing, China). Similarly, F-7000 fluorescence spectrophotometer (Hitachi, Japan) was arrayed for the photoluminescence studies. In addition, CdTe/ CdS/ZnS quantum dots were characterized by transmission electron microscope (TEM, Tecnai G2 20S-Twin, FEI Company, USA) at 200 kV. While, Bruker spectrometer (ESP-300E, Bruker, Germany) was deployed for EPR analysis.
2.3. CL testsLight-producing reactions were carried out in the glass cuvette by a batch method and the detection was performed on a BPCL luminescence analyzer under ambient conditions with 0.1 s interval at -1.2 kV. 50 μL CdTe/CdS/ZnS QDs solution was added into a cuvette, and 50 μL KIO4 solution was injected from the upper injection port subsequently by using a microliter syringe.
3. Results and discussion 3.1. Characterization of CdTe/CdS/ZnS QDsA commercial CdTe/CdS/ZnS QDs, with core/shell/shell structure, were applied to subsequent CL experiments. The CdTe/CdS/ ZnS QDs were modified with -COOH on the surface and possessed excellent solubility in water. The TEM study showed that the average size of CdTe/CdS/ZnS QDs was approximately 5 nm (Fig. 1a). Besides, as was shown in Fig. 1b, the PL spectra of CdTe/CdS/ZnS QDs were displayed, which revealed excellent photoresponsivity and a maximum emission at 616 nm.

|
Download:
|
| Figure 1. (a) The TEM image of CdTe/CdS/ZnS QDs. The corresponding size histogram was given aide. The concentration of CdTe/CdS/ZnS QDs was 5 μmol/L. (b) The PL spectra of KIO4-CdTe/CdS/ZnS QDs system. Black: excitation spectrum at a maximum emission of 616 nm. Different colors: emission spectra at different excitation wavelengths. The emission intensity increases from 340 nm to 540 nm, then decreases till 580 nm. The concentration of CdTe/CdS/ZnS QDs was 0.05 μmol/L. | |
3.2. Batch analysis
In CL study, KIO4-CdTe/CdS/ZnS QDs system was analyzed, as was shown in Fig. 2. Strong and fast CL performance appeared when KIO4 was injected into CdTe/CdS/ZnS QDs while no signal was observed when H2O was injected into CdTe/CdS/ZnS QDs instead (Fig. 2a). In addition, CdTe/CdS/ZnS QDs solution with red color turned white immediately as KIO4 was injected, which indicated that reaction indeed occurred during the mixing process.
|
Download:
|
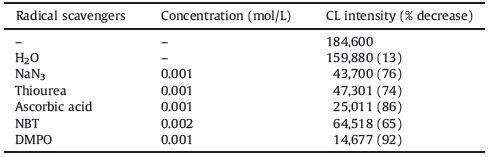
| Figure 2. (a) CL curves of different systems: (1) KIO4-QDs system; (2) H2O-QDs system as a control group. The concentration of KIO4 was 0.01 mol/L while that of QDs was 0.5 μmol/L. (b) CL curves under different CdTe/CdS/ZnS QDs concentration: (1) 0.5 nmol/L; (2) 5 nmol/L; (3) 0.05 μmol/L; (4) 0.5 μmol/L. The concentration of KIO4 was 0.01 mol/L. (c) CL curves with different KIO4 concentration: (1) 10-7 mol/L; (2) 10-6 mol/L; (3) 10-5 mol/L; (4) 10-4 mol/L; (5) 10-3 mol/L; (6) 10-2 mol/L. The concentration of CdTe/CdS/ZnS QDs was 0.5 μmol/L. (d) CL curves with different pH: (1) (14) represents pH from 1 to 14. The concentration of KIO4 was 0.01 mol/L while the concentration of QDs was 0.5 μmol/L. The volumes of all reagents were 50 μL. | |
Besides, effects of concentration and pH on the CL system were investigated. The concentrations of CdTe/CdS/ZnS QDs from the range of 0.5 nmol/L to 500 nmol/L were considered. No obvious CL signal was detected with concentration below 5 nmol/L while the CL intensity would be gradually intensified with the concentration of CdTe/CdS/ZnS QDs from 5 to 500 nmol/L (Fig. 2b). Meanwhile, the response of the system to different concentration of KIO4 was also studied, showing that the CL intensity increased with concentration in the range of 10-4 to 10-2 mol/L (Fig. 2c). As far as pH was concerned, high CL intensity was achieved with pH from 6-10, while both edges exhibited weak intensity (Fig. 2d).
3.3. Free radical studiesTo further study the mechanisms of the CL system, the effects of different active oxygen radical scavengers on the CL intensity of KIO4-CdTe/CdS/ZnS QDs system were investigated (Table 1) . NBT could be reduced by ·O2- anionic radicals to its deep-blue diformazan form and was frequently applied to the detection of ·O2-. An obvious decrease was observed in relevant CL experiment, and it could arrive a conclusion that ·O2- was generated in the system. NaN3 was a physical quencher for 1O2 in the CL system. The CL intensity was effectively quenched by NaN3, which further indicated that 1O2 might be involved in the CL reaction. The hydroxide radical was considered to be one of the most potent oxidizers. Thiourea and DMPO were effective radical scavengers for OH. The CL intensity was significantly decreased by thiourea or DMPO, which showed that .OH existed in the CL system. Besides, ascorbic acid as a classical reducing agent, was dehydrogenized by ROS to form dehydroascorbic acid and could be mostly employed as a common free radical scavenger. The decrease in CL intensity supported that the CL reaction ensured via radical pathway.
|
|
Table 1 Effect of free radical scavengers on KIO4-CdTe/CdS/ZnS QDs system. The concentration of KIO4 was 0.01 mol/L while that of CdTe/CdS/ZnS QDs was 0.5μmol/L. |
In summary, free radical scavenger studies showed that ·O2-, 1O2 and ·OH species were generated in the system. These radical species contributed to the CL boosting.
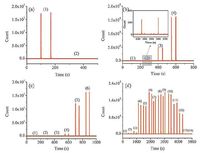
3.4. ESR studyRoom temperature ESR analysis was carried out to detect radical intermediates generated in the system. TEMP as a specific target for 1O2, reacted with it to yield the adduct 2, 2, 6, 6-tetramethyl-4-piperidine-N-oxide (TEMPO) that was a stable nitroxide radical with a distinctive spectrum. As was shown in Fig. 3a, negligible signal was observed when only TEMP was used for ESR analysis as background. Besides, weak signal was detected in mixture of QDs/TEMP while stronger signal could be obtained for KIO4/TEMP. It indicated that 1O2 existed in QDs and KIO4 solution, respectively. However, negligible signal was found in QDs/TEMP/KIO4 system. Instantaneous oxidation-reduction reaction occurred when QDs were mixed with KIO4 solution. Therefore, 1O2 were unavailable to bind with TEMP to give their characteristic band in EPR spectrum.

|
Download:
|
| Figure 3. ESR spectra of (a) 1O2 and (b) OH radicals. The concentration of KIO4 was 0.01 mol/L while the concentration of CdTe/CdS/ZnS QDs was 0.5 μmol/L. | |
Moreover, DMPO, was used to detect the production of DMPO/ .OH adducts in the studied system. Fig. 3b showed that DMPO, QDs/DMPO, and KIO4/DMPO were almost exhibited no signal in ESR analysis while high signal was obtained in mixture of QDs/ DMPO/KIO4. The results confirmed the generation of .OH in KIO4-CdTe/CdS/ZnS QDs CL system.
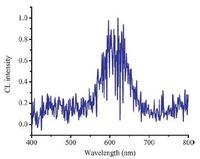
3.5. CL spectraTo illustrate the primary CL mechanism and identify the intermediate species in KIO4-CdTe/CdS/ZnS QDs system, CL emission spectrum was performed using fluorescence spectrophotometer, with a flow-injection system, without light source (Fig. 4) . It could be discovered that only one maximum emission peak centered around 620 nm was obtained, which was consistent with the PL spectrum of CdTe/CdS/ZnS QDs. Therefore, we presumed that the light emission in the CL system originated from CdTe/CdS/ZnS QDs.
|
Download:
|
| Figure 4. CL spectra of KIO4-CdTe/CdS/ZnS QDs system. The concentration of KIO4 was 0.01 mol/L while that of CdTe/CdS/ZnS QDs was 0.05 μmol/L. The flow rate of each reagent was 3 μL/min. | |
3.6. Mechanism
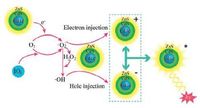
Based on the above research on KIO4-CdTe/CdS/ZnS QDs system, the possible CL mechanism could be illustrated in Scheme 1. The possible mechanism for QDs in an enhanced CL was that QDs were emitter species after direct oxidation.
|
Download:
|
| Scheme1. Schematic illustration of the CL mechanism in the KIO4-CdTe/CdS/ZnS QDs system. | |
For the direct oxidation mechanism, O2- could be generated from two avenues. One was from the dissolved oxygen in the solution by accepting an electron from QD [33] (reaction (1) ). The other way was from the reaction of IO4- and the dissolved oxygen in basic solution [34] (reaction (2) ). The produced O2- was unstable in solution, and could be easily transformed into singletoxygen and H2O2 [35] (reaction (3) ). Furthermore, the evolved O2·- and H2O2 leaded to the production of .OH [36] (reaction (4) ). Then QD (e-1 se) and QD (h1sh+) excitons were formed via electron injection and hole injection from O2- and .OH respectively (reaction (5) ) and (6) ), both of which were combined to generate QD*. Finally, QD* returned to the ground-state with CL emission (reaction (7) ) [37].
| $\text{QD + }{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\to \text{ QD }\!\!\cdot\!\!\text{ + +}{{\text{O}}_{\text{2}}}^{\text{ }\!\!\cdot\!\!\text{ -}}\text{ }\!\!~\!\!\text{ }\left[ \text{31} \right]$ | (1) |
| $\text{I}{{\text{O}}_{\text{4}}}^{\text{-}}\text{ }\!\!~\!\!\text{ +}{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ +2OH- }\to \text{ 2}{{\text{O}}_{\text{2}}}^{\text{ }\!\!\cdot\!\!\text{ -}}\text{ }\!\!~\!\!\text{ +I}{{\text{O}}_{\text{3}}}\text{ }\!\!~\!\!\text{ - +}{{\text{H}}_{\text{2}}}\text{O}\left[ \text{32} \right]$ | (2) |
| ${{\text{O}}_{\text{2}}}^{\text{ }\!\!\cdot\!\!\text{ -}}\text{ }\!\!~\!\!\text{ +}{{\text{O}}_{\text{2}}}^{\text{ }\!\!\cdot\!\!\text{ -}}\text{ }\!\!~\!\!\text{ +2}{{\text{H}}_{\text{2}}}\text{O }\to {{\text{ }\!\!~\!\!\text{ }}^{\text{1}}}{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ +}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ +2OH- }\left[ \text{33} \right]$ | (3) |
| ${{\text{O}}_{\text{2}}}^{\text{ }\!\!\cdot\!\!\text{ -}}\text{ }\!\!~\!\!\text{ +}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\to \text{ }{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ + }\!\!\cdot\!\!\text{ OH + OH-}\left[ \text{34} \right]$ | (4) |
| ${{\text{O}}_{\text{2}}}^{\text{ }\!\!\cdot\!\!\text{ -}}\text{ }\!\!~\!\!\text{ +QD }\to \text{ QD}\left( {{\text{e}}^{\text{- }}}_{\text{1se}} \right)\text{ + }{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\left[ \text{35} \right]$ | (5) |
| $\text{OH + QD }\to \text{ QD}\left( {{\text{h}}^{\text{+}}}{{\text{ }}_{\text{1sh}}} \right)\text{ + OH- }\left[ \text{35} \right]$ | (6) |
| $\text{QD}\left( {{\text{e}}^{\text{-}}}{{\text{ }}_{\text{1se}}} \right)\text{ + QD}\left( {{\text{h}}^{\text{+}}}{{\text{ }}_{\text{1sh}}} \right)\text{ }\to \text{ QD* }\to hv\text{ }\left[ \text{35} \right]$ | (7) |
Furthermore, the state of CdTe/CdS/ZnS QDs before and after the CL reactions was also examined by PL and IR analysis. It could
be observed that the characteristic PL emission peak of CdTe/CdS/ ZnS QDs disappeared after the CL reaction (Fig. 5a). Besides, IR studies also showed that difference in composition existed (Fig. 5b). The presence stretching of S-CH2 at 1400 cm-1, C=O bond at 1546 cm-1 and O-H bond at 3418 cm-1 indicated the formation of the capping layer over the CdTe/CdS/ZnS QDs. However, absence of peaks in 2500-2900 cm-1 after addition of KIO4 solution was possibly resulted from the cleavage of -SH. These demonstrated that the structure of CdTe/CdS/ZnS QDs might be damaged after direct oxidation by KIO4. These two spectra also supported the mechanism deduced previously.

|
Download:
|
| Figure 5. (a) The PL spectra of CdTe/CdS/ZnS QDs and its mixture with KIO4. 1: before mixing; 2: after mixing. The concentration of KIO4 was 0.01 mol/L while that of CdTe/CdS/ ZnS QDs was 0.5 μmol/L. (b) IR spectra of CdTe/CdS/ZnS QDs (black) and its mixture with KIO4 (red). The concentration of KIO4 was 0.01 mol/L while that of CdTe/CdS/ZnS QDs was 5 μmol/L. | |
4. Conclusion
In this work, a novel CL system of CdTe/CdS/ZnS QDs and KIO4 was studied. Strong and fast CL performance appeared when KIO4 solution was injected into CdTe/CdS/ZnS QDs directly while no signal was observed when H2O was employed instead. This performance could be attributed to direct oxidation, within which the excited state of CdTe/CdS/ZnS QDs was evolved as emitter species. Relevant CL mechanism was speculated to be radiative recombination of injected holes and electron. The established KIO4-CdTe/CdS/ZnS QDs CL system broadened the scopes of traditional QDs-based CL analysis and contributed to a better understanding of CL mechanism. This investigation not only opened new sight into the optical characteristics of CdTe/CdS/ZnS QDs, but also expanded the conventional optical utilizations of QDs. Besides, it was believed that this new CL system with excellent intensity and reproducibility could be applied to concentration detection of specific compounds.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 81373373, 21435002, 21227006) .
| [1] | A.P. Alivisatos, Semiconductor clusters, nanocrystals, and quantum dots. Science 271 (1996) 933–937. DOI:10.1126/science.271.5251.933 |
| [2] | T.M. Jovin, Quantum dots finally come of age. Nat. Biotechnol. 21 (2003) 32–33. DOI:10.1038/nbt0103-32 |
| [3] | J.M. Klostranec, W.C.W. Chan, Quantum dots in biological and biomedical research:recent progress and present challenges. Adv. Mater. 18 (2006) 1953–1964. DOI:10.1002/(ISSN)1521-4095 |
| [4] | D. Mocatta, G. Cohen, J. Schattner, Heavily doped semiconductor nanocrystal quantum dots. Science 332 (2011) 77–81. DOI:10.1126/science.1196321 |
| [5] | E.A. Stinaff, M. Scheibner, A.S. Bracker, Optical signatures of coupled quantum dots. Science 311 (2006) 636–639. DOI:10.1126/science.1121189 |
| [6] | H.T. Li, X.D. He, Z.H. Kang, Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 49 (2010) 4430–4434. DOI:10.1002/anie.200906154 |
| [7] | X.X. Chen, Q.Q. Jin, L.Z. Wu, C. Tung, X.J. Tang, Synthesis and unique photoluminescence properties of nitrogen-rich quantum dots and their applications. Angew. Chem. Int. Ed. 53 (2014) 12542–12547. |
| [8] | J.H. Warner, A. Hoshino, K. Yamamoto, X.J. Tang, Water-soluble photoluminescent silicon quantum dots. Angew. Chem. Int. Ed. 44 (2005) 4550–4554. DOI:10.1002/(ISSN)1521-3773 |
| [9] | R.J. Ellingson, M.C. Beard, J.C. Johnson, Highly efficient multiple exciton generation in colloidal PbSe and PbS quantum dots. Nano Lett. 5 (2005) 865–871. DOI:10.1021/nl0502672 |
| [10] | Y. Wang, L. Zhang, R.P. Liang, J.M. Bai, J.D. Qiu, Using graphene quantum dots as photoluminescent probes for protein kinase sensing. Anal. Chem. 85 (2013) 9148–9155. DOI:10.1021/ac401807b |
| [11] | V. Biju, T. Itoh, M. Ishikawa, Delivering quantum dots to cells:bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 39 (2010) 3031–3056. DOI:10.1039/b926512k |
| [12] | X.L. Dai, Z.X. Zhang, Y.Z. Jin, Solution-processed, high-performance lightemitting diodes based on quantum dots. Nature 515 (2014) 96–99. DOI:10.1038/nature13829 |
| [13] | R.J. Forster, P. Bertoncello, T.E. Keyes, Electrogenerated chemiluminescence. Annu. Rev. Anal. Chem. 2 (2009) 359–385. DOI:10.1146/annurev-anchem-060908-155305 |
| [14] | W. Adam, D.V. Kazakov, V.P. Kazakov, Singlet-oxygen chemiluminescence in peroxide reactions. Chem. Rev. 105 (2005) 3371–3387. DOI:10.1021/cr0300035 |
| [15] | C. Dodeigne, L. Thunus, R. Lejeune, Chemiluminescence as diagnostic tool. A review. Talanta 51 (2000) 415–439. DOI:10.1016/S0039-9140(99)00294-5 |
| [16] | L.J. Kricka, Clinical applications of chemiluminescence. Anal. Chim. Acta 500 (2003) 279–286. DOI:10.1016/S0003-2670(03)00809-2 |
| [17] | M. Iranifam, Analytical applications of chemiluminescence methods for cancer detection and therapy. TrAC Trend Anal. Chem. 59 (2014) 156–183. DOI:10.1016/j.trac.2014.03.010 |
| [18] | K. Tsukagoshi, N. Jinno, R. Nakajima, Development of a micro total analysis system incorporating chemiluminescence detection and application to detection of cancer markers. Anal. Chem. 77 (2005) 1684–1688. DOI:10.1021/ac040133t |
| [19] | M. Mirasoli, E. Michelini, Analytical bioluminescence and chemiluminescence. Anal. Bioanal. Chem. 406 (2014) 5529–5530. DOI:10.1007/s00216-014-7992-4 |
| [20] | A. Roda, M. Guardigli, P. Pasini, M. Mirasoli, Bioluminescence and chemiluminescence in drug screening. Anal. Bioanal. Chem. 377 (2003) 826–833. DOI:10.1007/s00216-003-2096-6 |
| [21] | S.W. Qi, Q.L. Li, W. Rao, Determining the concentration of procalcitonin using a magnetic particles-based chemiluminescence assay for the clinical diagnosis of sepsis. Anal. Sci. 29 (2013) 805–810. DOI:10.2116/analsci.29.805 |
| [22] | A. Myint, Q.L. Zhang, L.J. Liu, H. Cui, Flow injection-chemiluminescence determination of paraben preservative in food safety. Anal. Chim. Acta 517 (2004) 119–124. DOI:10.1016/j.aca.2004.04.044 |
| [23] | S.K. Poznyak, D.V. Talapin, E.V. Shevchenko, H. Weller, Quantum dot chemiluminescence. Nano Lett. 4 (2004) 693–698. DOI:10.1021/nl049713w |
| [24] | H. Chen, L. Lin, H.F. Li, J.M. Lin, Quantum dots-enhanced chemiluminescence:mechanism and application, Coord. Chem. Rev. 263-264(2014) 86-100. |
| [25] | L.Q. Song, J.Q. Shi, J. Lu, C. Lu, Structure observation of graphene quantumdots by single-layered formation in layered confinement space. Chem. Sci. 6 (2015) 4846–4850. DOI:10.1039/C5SC01416F |
| [26] | Y.S. Zhao, S.L. Zhao, J.M. Huang, F.G. Ye, Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 85 (2011) 2650–2654. DOI:10.1016/j.talanta.2011.08.032 |
| [27] | L.X. Zhao, F. Di, D.B. Wang, Chemiluminescence of carbon dots under strong alkaline solutions:a novel insight into carbon dot optical properties. Nanoscale 5 (2013) 2655–2658. DOI:10.1039/c3nr00358b |
| [28] | L.X. Zhao, F.L. Geng, F. Di, Polyamine-functionalized carbon nanodots:a novel chemiluminescence probe for selective detection of iron (III) ions. RSC Adv. 4 (2014) 45768–45771. DOI:10.1039/C4RA08071H |
| [29] | Z. Lin, W. Xue, H. Chen, J.M. Lin, Classical oxidant induced chemiluminescence of fluorescent carbon dots. Chem. Commun. 48 (2011) 1051–1053. |
| [30] | Y.R. Tang, Y.Y. Su, N. Yang, L.C. Zhang, Y. Lv, Carbon nitride quantum dots:a novel chemiluminescence system for selective detection of free chlorine in water. Anal. Chem. 86 (2014) 4528–4535. DOI:10.1021/ac5005162 |
| [31] | Z.F. Ding, B.M. Quinn, S.K. Haram, Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science 296 (2002) 1293–1297. DOI:10.1126/science.1069336 |
| [32] | J.X. Liu, H. Chen, L. Lin, C. Lu, J.M. Lin, Sensitized chemiluminescence reaction between hydrogen peroxide and periodate of different types of Mn-doped ZnS quantum dots. Chin. Sci. Bull. 55 (2010) 3479–3484. DOI:10.1007/s11434-010-4059-6 |
| [33] | X.G. Dou, Z. Lin, H. Chen, Production of superoxide anion radicals as evidence for carbon nanodots acting as electron donors by the chemiluminescence method. Chem. Commun. 49 (2013) 5871–5873. DOI:10.1039/c3cc41145a |
| [34] | J.M. Lin, M. Yamada, Oxidation reaction between periodate and polyhydroxyl compounds and its application to chemiluminescence. Anal. Chem. 71 (1999) 1760–1766. DOI:10.1021/ac981341m |
| [35] | Y.Z. Zheng, X.G. Dou, H.F. Li, J.M. Lin, Bisulfite induced chemiluminescence of g-C3N4 nanosheets and enhanced by metal ions. Nanoscale 8 (2016) 4933–4937. DOI:10.1039/C5NR08943C |
| [36] | H. Chen, C. Lu, R.B. Li, G.S. Guo, J.M. Lin, Chemiluminescence behavior of sodium hydrogen carbonate in the potassium permanganate-hydrogen peroxide reaction. Sci. China Chem. 53 (2010) 1784–1792. |
| [37] | Z.P. Wang, J. Li, B. Liu, Chemiluminescence of CdTe nanocrystals induced by direct chemical oxidation and its size-dependent and surfactant-sensitized effect. J. Phys. Chem. B 109 (2005) 23304–23311. DOI:10.1021/jp055023k |
 2017, Vol. 28
2017, Vol. 28