b College of Chemistry, Beijing Normal University, Beijing 100875, China
As one of the major indispensable substances in life activities, glucose offers 70% of the energy requirement and plays crucial role in the composition of tissue and liver protection [1]. For reliable monitoring of glucose, non-enzymatic glucose sensors relied on metal or carbon based materials exhibit better stability towards variation of environment [2, 3]. And carbon structures can play an increasingly critical role in nanoelectronics and nanobiological devices due to their unique geometry. Specifically, when these carbon-based nanocomposites are used as active materials for glucose sensor, the carbon substrate in the composites plays an important role in increasing surface areas and improving conductivity, and thus leading enhanced electrochemical performances. In recent years, various strategies have been developed to fabricate the carbon-based composites for glucose sensor. For example, Xiang et al. synthesized ordered mesoporous carbon through ordered SBA-15 as a template, and then embedded by NiFex alloy via a wet impregnation process [4]. Hwa et al. prepared graphene-carbon nanotube-ZnO composite by zinc acetate reduction in graphene-carbon nanotube solution [5]. Although some development has been made by researchers, the design of the novel carbon-based composites with more active sites and higher conductivity is highly desirable for the electrocatalytic improvement, especially a simple and scaled-up method is still urgent needed.
In this paper, we present NiAl-LDH decorated CMC by combining C-MEMS technology [6, 7] with in situ growth progress. CMC was synthesized based on the photolithography of SU-8 resist and following pyrolysis procedure. NiAl-LDH/CMC was then fabricated by in situ growth of NiAl-LDH on obtained CMC. NiAlLDH/CMC modified glass carbon electrodes (GCE) exhibited great electrical performance towards glucose. During testing, each single hybrid microcylinder modified on the surface of the GC can work as independent testing unit for glucose detection. With the advantages of low cost, high uniformity and electoactivities, this work can guide the design of electroactive micro materials for mass production.
2. ExperimentalThe detailed materials preparation is available in Supporting information.
3. Results and discussionThe synthetic procedure is shown as Fig. 1. In the first step, a film of SU-8 2050 photoresist was coated on the Si wafer by spinning on a SC-1B spin coater, when the film was exposed on the ultraviolet rays through the patterning mask and rinsed with SU-8 developer, the exposed orderly matrix was obtained. In order to further fabricate the carbon microcylinders, the matrix then pyrolysis in the inert gas environment. In the second step, the obtained matrix of carbon microcylinder was coated by the Al (OOH) sol and then the NiAl-LDH/CMC was finally fabricated after hydrothermal reaction. Noteworthily, according to the literature, the carbon obtained from pyrolyzation of photoresisit shows glassy carbon like properties with high conductivity and very low background current, this could be a great advantage for electroanalytic application [8]. Therefore, when the 3D macroporous NiAl-LDH film modified on the carbon substrate, a large surface area and great electronic/ionic conductivity between CMC and NiAl-LDH would be obtained, which benefit for the glucose detection.
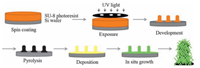
|
Download:
|
| Figure 1. Schematic representation of preparation of the NiAl-LDH/CMC microelectrode. | |
With proper control of process parameters, NiAl-LDH/CMC samples were fabricated. And the morphology of NiAl-LDH/CMC samples in different stage was characterized by scanning electron microscopy (SEM) technique. Firstly, Fig. 2A displays the SEM image of the as-prepared orderly microcylinder matrix, it can be seen that the carbon microarrays were successfully obtained by the C-MEMS fabrication technology, and the distance between two cylinders is about~60 μm. In addition, a higher magnification SEM image shown in Fig. 2A inset reveals the diameter of microcylinder is~30 μm and the length is about~55 μm. Meaningfully, the diameter of microcylinder and the distance between them are decided by the patterning mask used in the experiment, which can be easily tuned in the scale-up production [9]. What’s more, due to relatively larger diameter, small length and suitable distance between two cylinders, this array provides a promising vehicle for later surface deposition and in situ growth process. A thin film of AlOOH coated right along the surface of the microcylinder is shown in Fig. 1B. From the SEM image, we can clearly observe that an amorphous film was coated on the microcylinder. Especially from the high magnification image (inset Fig. 2B), the thickness of the AlOOH film is about~1 μm. Subsequently, the LDH film was grown along the surface of microcylinder with the AlOOH film as the seed layer, and Fig. 2C shows the typical SEM images of the hybrid CMC arrays. It was observed that the morphology of asprepared CMC arrays was replicated and the 3D macroporous NiAlLDH/CMC was clearly discovered. Significantly, large quantity of NiAl-LDH is directly grown onto the surface of CMC, and curved NiAl-LDH sheet forms a dense coverage of porous LDH film along the surface, offers large amount of electrocatalytic active sites. Therefore, the excellent electrocatalytic activity should be obtained.

|
Download:
|
| Figure 2. (A) SEM images of the orderly matrix of carbon microcylinder, (B) SEM images of the AlOOH/CMC, (C) SEM images of the NiAl-LDH/CMC, (D) SEM images of the NiAlLDH/CMC/GCE, (E) Schematic illustration of the diffuse reflection during exposure, (F) XRD patterns of the NiAl-LDH/CMC composite material. | |
Since the NiAl-LDH/CMC was removed from the substrate and modified on the GCE by a simple drop casting method (see Supporting information for more details), it is important to investigate the arrangement of the composite material on the testing working electrode. Therefore, the surface feature of the modified GCE with NiAl-LDH/CMC was observed in Fig. 2D. The SEM image show that most of the NiAl-LDH/CMC were immobilized in standing position, which is a best positon to expose maximum electroactive sites, and this is due to the irregular shape of the NiAl-LDH/CMC composite. Fig. 2E clearly describes the formation reason of this irregular shape of NiAl-LDH/CMC composite. As we know, one of the advantages of C-MEMS technology is with proper control, and the parameters of all experiment process can be tuned to synthesize various shaped materials. In this experiment, matching the light intensity is an excellent choice. When the light intensity was increased, the UV light can be reflected by the Si wafer, a part of bottom photoresist was also solidified which induced by the reflected light. Therefore, the irregular shaped microcylinders with large area of bottom side (inset Fig. 2D) were obtained. Interestingly, due to the irregular larger area of bottom side, the NiAl-LDH/CMC composites are more likely to stand on its bottom side during electrode modification process. The typical XRD pattern of the NiAl-LDH/CMC is shown in Fig. 2F. The sharp peaks at 2θ values of 11.55° and 23.34° can be assigned to the (0 0 3) and (0 0 6) reflections of LDH crystals, respectively [10], indicating the well formation of a hydrotalcitelike LDH phase. Since the CMC made by pyrolysis of photoresist is amorphous carbon material, it is also observed that there is no significant diffraction peak from crystal structure of carbon. Considering the great electrocatalytic activity of NiAl-LDH film and outstanding electronic conductivity of CMC, the excellent glucose detection performance should be achieved.
Fig. 3A shows the CVs of the NiAl-LDH/CMC/GCE in 0.1 mol L-1 NaOH (pH 13) in the absensce and presence of 10 mmol L-1 of glucose at a scan rate of 0.05 V s-1. It is seen that the NiAl-LDH/ CMC/GCE exhibit a pair of well-defined anodic and cathodic peaks, which are attributed to the redox reaction of Ni2+/Ni3+ couple on the electrode surface [11]. After addition of 10 mmol L-1 glucose, the intensity of oxidation peak shows a small increase, indicating the fabricated NiAl-LDH/CMC can catalyze oxidation of glucose in alkaline solution.

|
Download:
|
| Figure 3. (A) CVs of the NiAl-LDH/CMC/GCE recorded in 0.1 mol L-1 NaOH solution (a) and in NaOH solution with 10 mmol L-1 glucose (b). (B) Typical current-time responses of the NiAl-LDH/CMC/GCE to successive addition of glucose in a stirred 0.1 mol L-1 NaOH. (C) Plot of the steady-state current of the NiAl-LDH/CMC/GCE as a function of glucose concentration. | |
Moreover, the amperometric current-time method was used to investigate the effect of NiAl-LDH/CMC/GCE (modified with 2 mg mL-1 of microelectrodes, so did the GCE in later experiment) on the catalytic activity of glucose at 0.48 V in a 0.1 mol L-1 NaOH aqueous solution. As shown in Fig. 3B, with addition of glucose into the stirring solution, the catalytic current increased rapidly and a regular stair-stepped curve was obtained. The fabricated sensor exhibited a linear range of glucose detection from 0.2 mmol L-1 to 18.6 mmol L-1 with a high correlation coefficient of R=0.999, and a sensitivity of 0.125 μA·(mmol L-1)-1, detection limit of 0.12 mmol L-1 (Fig. 3C).
To determine NiAl-LDH/CMC/GCE for practical applications, it was applied to examine glucose concentration in human blood serum. Amperometric detection was carried out with the serum sample as the target analyte using the standard three electrodes system. Table 1 shows the comparison of measurements obtained from the modified electrode and those results from the automatic biochemistry analyzer in local hospital. It can be seen that prepared electrode shows the measuring relative standard deviation (RSD) less than 2%, and the accuracy with deviation less than 6%. The satisfying results implied that the prepared NiAlLDH/CMC/GCE is reliable for the determination of glucose in real samples. The overall performance reveals that the NiAl-LDH/CMC/ GCE represents an effective sensor for glucose measurement, which elucidates the potential application of NiAl-LDH/CMC microcylinder in micro non-enzymatic sensors.
|
|
Table 1 Testing results of glucose concentration in human blood serum. |
Overall, the superior characteristics of the modified electrode towards the oxidation of glucose can be assigned to the advantageous features of the structure of the NiAl-LDH/CMC: first, curved NiAl-LDH sheet formed a dense coverage of porous LDH film growing along the surface of each carbon microcylinder, and offered large effective surface area for electrocatalytic detection; second, carbon microcylinder based on pyrolysis of lithographic patterns makes an excellent current collector owning to low background current and high conductivity [12].
4. ConclusionIn summary, we offered a simple and practical method for fabrication of NiAl-LDH/CMC microcylinder based on typical lithography techniques combined with in situ growth progress, and constructed glucose sensor with the microelectrode modified GCE. The electrochemical behavior showed that the fabricated sensor displays a good superiority in term of linear range, sensitivity, selectivity, reproducibility and stability. With the low cost and uniform property, this strategy could be a promising candidate for future functional applications, such as micro sensors, energy storage, and so on.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21574014), [1_TD$DIF] the Inner Mongolia University of Science and Technology Innovation Fund (2016QDL-B05) and by Shenzhen Rongda Photosensitive Science and Technology Co., Ltd., China.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.023.
| [1] | Y. Xiang, Y. Lu, Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 3 (2011) 697–703. DOI:10.1038/nchem.1092 |
| [2] | B. Hai, Y.Q. Zou, Carbon cloth supported NiAl-layered double hydroxides for flexible application and highly sensitive electrochemical sensors. Sens. Actuators B 208 (2015) 143–150. DOI:10.1016/j.snb.2014.11.022 |
| [3] | W.W. Tang, L. Li, X.P. Zeng, A glucose biosensor based on the synergistic action of nanometer-sized TiO2 and polyaniline. Talanta 131 (2015) 417–423. DOI:10.1016/j.talanta.2014.08.019 |
| [4] | D. Xiang, L.W. Yin, J.Y. Ma, Amperometric hydrogen peroxide and glucose biosensor based on NiFe2/ordered mesoporous carbon nanocomposites. Analyst 140 (2015) 644–653. DOI:10.1039/C4AN01549E |
| [5] | K.Y. Hwa, B. Subramani, Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 62 (2014) 127–133. DOI:10.1016/j.bios.2014.06.023 |
| [6] | J.W. Liu, C. Bian, J.H. Han, S.F. Chen, S.H. Xia, A silicon-based bulk micromachined amperometric microelectrode biosensor with consecutive platinization and polymerization of pyrrole. Sens. Actuators B 106 (2005) 591–601. DOI:10.1016/j.snb.2004.07.027 |
| [7] | H. Xu, K. Malladi, C.L. Wang, Carbon post-microarrays for glucose sensors. Biosens. Bioelectron. 23 (2008) 1637–1644. DOI:10.1016/j.bios.2008.01.031 |
| [8] | S. Ranganathan, R.L. McCreery, Electroanalytical performance of carbon films with near-atomic flatness. Anal. Chem. 73 (2001) 893–900. DOI:10.1021/ac0007534 |
| [9] | C.L. Wang, M. Madou, From MEMS to NEMS with carbon. Biosens. Bioelectron. 20 (2005) 2181–2187. DOI:10.1016/j.bios.2004.09.034 |
| [10] | Y.F. Zhao, S. He, M. Wei, D.G. Evans, X. Duan, Hierarchical films of layered double hydroxides by using a sol-gel process and their high adaptability in water treatment. Chem. Commun. 46 (2010) 3031–3033. DOI:10.1039/b926906a |
| [11] | S. Liu, B. Yu, T. Zhang, A novel non-enzymatic glucose sensor based on NiO hollow spheres. Electrochim. Acta 102 (2013) 104–107. DOI:10.1016/j.electacta.2013.03.191 |
| [12] | S. Ranganathan, R. McCreery, S.M. Majji, M. Madou, Photoresist-derived carbon for microelectromechanical systems and electrochemical applications. J. Electrochem. Soc. 147 (2000) 277–282. DOI:10.1149/1.1393188 |
 2017, Vol. 28
2017, Vol. 28 



