b Dyal Singh College, University of Delhi, Lodhi Road, Delhi 110003, India;
c Department of Dairy Microbiology, College of Dairy Science and Technology, Guru Angad Dev Veterinary and Animal Science University, Ludhiana 141004, India
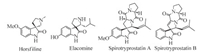
Infectious diseases caused by microbes such as bacteria and fungi are increasing and the major reason for the increase in microbial infections is the resistance developed by these microbial organisms towards existing antimicrobial drugs [1]. Therefore, development of alternative new more effective antimicrobial agents with new modes of action and a broad spectrum of activities is a major challenge. Spirooxindoles have played an important role in the field of medicinal chemistry (Fig. 1). These heterocyclic compounds with spirooxindole and oxindole framework are endowed with a wide range of biological activities such as analgesic, fungicidal, antidepressant, antitumor, antibiotic [2, 3], potent inhibition of monoamine oxidase (MAO) in human urine and rat tissues [4], inhibition of several enzymes such as acetylcholinestrease (AChE) and atrial natriuretic peptide-stimulated guanylate cyclase [5] and potent antagonist of in vitro receptor binding by atrial natriuretic peptide [6], besides possessing a wide range of central nervous system activities [7]. Isatin and its derivatives are very useful synthetic skeletons for the synthesis of a large number of spirocyclic compounds via MCRs because of their high atom economy, operational simplicity and efficiency [8]. Isatin containing compounds also exhibit a variety of biological activities [9].
|
Download:
|
| Figure 1. Some biologically active compounds containing spirooxindole core structure. | |
Synthesis of spiro[indolo-3, 100-indeno[1, 2-b]quinolin]-2, 4, 110-triones has been reported recently using CTAB [10] and La (OTf)3 [11] as the catalyst but long reaction time and expensive catalyst are the drawbacks of these methodologies. In continuation of our research interest in the synthesis of bioactive heterocyclic compounds by green methodologies [12], we decided to investigate newer protocol for the synthesis of spiro[indolo-3, 100-indeno[1, 2-b]quinolin]triones under mild conditions with high yields.
2. ExperimentalAll the reagents and solvents were commercially available and were used as received. Thin layer chromatography (GF254) was used to monitor reaction progress. Melting points were measured on Buchi M-560 melting point apparatus and are uncorrected. IR (CHCl3) spectra were recorded on perkin Elmer FTIR spectrophotometer and values are expressed nmax (cm-1). The 1H NMR and 13C NMR spectra were recorded on Jeol JNM ECX-400P at 400 and 100 MHz respectively, using TMS as internal standard. The chemical shift values are recorded on δ scale. Mass spectral data were recorded on Agilent 6200 QT of (ESI-HRMS) Mass Spectrometer. Cyclic enaminones were prepared by condensation of cyclic 1, 3-diketones with alkylamines according to the reported method [13].
2.1. General procedure for the synthesis of spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 110-triones (IVa-IVv)A solution of enaminone (1.0 mmol), indane 1, 3-dione (1.0 mmol) and isatin (1.0 mmol) in 5 mL of ethanol-water (1:1, v/v) was heated under reflux in the presence of catalytic amount of CAN (10 mol%) for the appropriate time as mentioned in Table 2. The progress of the reaction was monitored by TLC using ethyl acetate:petroleum ether (40:60, v/v) as eluent. After completion of the reaction, water (10 mL) was added to the reaction mixture and the solid obtained was filtered at pump. The crude products were then purified either by flash column chromatography (IVa-IVg) using 10% ethyl acetate in petroleum ether as eluent or by recrystallisation from acetone (IVh-IVt), to afford pure products as orange solids. All the products were characterized by m.p., IR, 1H NMR, 13C NMR and mass spectra, if required. The characterization data for the synthesized compounds is given in Supporting information.
|
|
Table 2 Synthesis of spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones (IVa-IVv)a |
2.2. Antimicribial assay 2.2.1. Test microorganisms
Total six microbial strains were selected on the basis of their clinical importance in causing diseases in humans. Two Grampositive bacteria (Staphylococcus aureus MTCC 96 and Bacillus subtilis MTCC 121), two Gram-negative bacteria (Escherichia coli MTCC 1652 and Pseudomonas aeruginosa MTCC 741) and two yeasts, Candida albicans (MTCC 227), and Saccharomyces cerevisiae (MTCC 170) were screened for evaluation of antibacterial and antifungal activity of the 16 compounds. All the microbial cultures were procured from Microbial Type Culture Collection (MTCC), IMTECH, Chandigarh. The bacteria were subcultured on Nutrient agar whereas yeast on Sabourauds dextrose agar.
2.2.2. Antimicrobial activity (bacteria and yeasts)The antimicrobial activity of chemical compounds was evaluated by the agar well diffusion method. All the microbial cultures were adjusted to 0.5 McFarland standard, which is visually comparable to a microbial suspension of approximately 1.5 × 108 cfu/mL. 20 mL of Agar medium was poured into each Petri plate and plates were swabbed with 100 mL inocula of the test microorganisms and kept for 15 min for adsorption. Using sterile cork borer of 8 mm diameter, wells were bored into the seeded agar plates and these were loaded with a 100 μL volume with concentration of 8.0 mg/mL of each compound reconstituted in the dimethyl sulphoxide (DMSO). All the plates were incubated at 37 ℃ for 24 h. Antimicrobial activity of each compound was evaluated by measuring the zone of growth inhibition against the test organisms with zone reader (HiAntibiotic zone scale). DMSO was used as a negative control whereas Ciprofloxacin was used as positive control for bacteria and amphotericin-B for yeast. This procedure was performed in three replicate plates for each organism [14].
2.2.3. Determination of minimum inhibitory concentration (MIC) of chemical compoundsMIC is the lowest concentration of an antimicrobial compound that will inhibit the visible growth of a microorganism after overnight incubation. MIC of the various compounds against bacterial and yeast strains was tested through a modified agar well diffusion method [14]. In this method, a two-fold serial dilution of each chemically synthesized compound was prepared by first reconstituting the compound in DMSO followed by dilution in sterile distilled water to achieve a decreasing concentration range of 512-1 μg/mL. A 100 μL volume of each dilution was introduced into wells (in triplicate) in the agar plates already seeded with 100 μL of standardized inoculum (106 cfu/mL) of the test microbial strain. All test plates were incubated aerobically at 37 ℃ for 24 h and observed for the inhibition zones. MIC, taken as the lowest concentration of the chemical compound that completely inhibited the growth of the microbe, showed by a clear zone of inhibition, was recorded for each test organism. Ciprofloxacin and amphotericin B was used as positive control while DMSO as negative control.
3. Results and discussion 3.1. ChemistryWe report in this paper, a new protocol for the synthesis of spiro[indolo-3, 100-indeno[1, 2-b]quinolin]-2, 4, 110-triones from enaminones, isatin and indane 1, 3-dione in EtOH-H2O (1:1) in presence of CAN under reflux. The reactions were complete in 10-40 min and gave the products in high yields by a simple workup. Reactions of 5, 5-dimethyl-3-(phenylamino) cyclohex-2-enone (1.0 mmol), indoline-2, 3-dione (1.0 mmol) and 1, 3-indandione (1.0 mmol) were attempted under different conditions in order to find out a novel protocol. Initially, a reaction of the above substrates was carried out in ethanol-water (1:1) in presence of DBU (10 mol%) under reflux and progress of the reaction was monitored by TLC using (ethyl acetate:petroleum ether, 40:60, v/v) as eluent. The reaction was complete in 35 min as monitored by TLC. After workup and separation, 60, 60-dimethyl-10-phenyl-60, 70-dihydrospiro[indeno[1, 2-b]quinoline-10, 30-indole]-20, 40, 11(1'H, 5H, 5'H)-trione (IVa) was obtained in 65% yield (Table 1, entry 1).
|
|
Table 1 Optimization of reaction conditions for the synthesis of 6', 6'-dimethyl-1'-phenyl-6', 7'-dihydrospiro[indeno[1, 2-b]quinoline-10, 3'-indole]-2', 4', 11(1'H, 5H, 5'H)-trionea |
The above reaction was then attempted in ethanol-water (1:1) with different catalysts such as FeCl3, piperidine, La2(CO3)3, La (NO3)2 and Et3N (10 mol% each). The reactions were complete in 120-180 min and furnished moderate yields of the desired product IVa after chromatographic separations (Table 1, entries 2-6). The reaction was then attempted with ceric ammonium nitrate (CAN) as catalyst (10 mol%) in ethanol-water (1:1, v/v) under reflux. The reaction was complete in 90 min as monitored by TLC and gave 86% yield of desired product (IVa) (Table 1, entry 7). The reaction catalyzed by CAN was then performed in different solvents such as water, water-acetonitrile, water-methanol and ethanol under otherwise identical conditions. The reactions were complete in 120-180 min and gave 49%-72% of the desired product, respectively (Table 1, entries 8-11).
Therefore, ethanol-water (1:1, v/v) under reflux using CAN as the catalyst was found to be suitable reaction condition for the synthesis of our desired products. All the results are listed in Table 1. The scope and generality for the synthesis of spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11-triones by the present protocol were then examined by attempting reactions of various enaminones (also tried with enaminoneof heterocyclic amine like2-furfuryl amine), substitutedisatins and indane 1, 3-dione in presence of CAN (10 mol%) as catalyst in ethanol-H2O (1:1, v/v) under reflux. All the reactions went to completion in 10-40 min and gave high yields of the corresponding spiro[indolo-3, 100-indeno[1, 2-b]quinolin]-2, 4, 11'-triones. All the results are listed in Table 2.
All the products were identified by m.p., IR, 1H NMR, 13C NMR and mass spectra, wherever required. The two-dimensional NMR spectra of HMBC and COSY correlations are useful in the signal assignment of 4f and various characteristic signals are shown in Fig. 2.
|
Download:
|
| Figure 2. HMBC and COSY correlations of IVf and various characteristic 1H NMR and 13C NMR peaks. | |
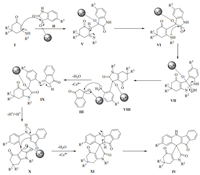
A probable mechanism involved in the formation of products is outlined in Scheme 1. The most exciting feature of mechanism is the unusual ring opening of an isatin moiety. At first, the nucleophilic addition reaction occurs between the enaminone (Ⅰ) with the more electrophilic carbonyl centre of isatin (Ⅱ) in Lewis acid medium (Ce4+ binds with carbonyl oxygen of isatin (Ⅱ) and enhances the electrophilic nature) to give an imine species (Ⅴ) that tautomerizes to yield an intermediate (Ⅵ). This intermediate (Ⅵ) undergoes intramolecular cyclisation to form the intermediate (Ⅶ), which is immediately converted to a more reactive and unstable intermediate (Ⅷ) via ring-opening of indoline-2, 3-dione. The ring opening takes place by nucleophilic attack of nitrogen to amidic carbonyl carbon of isatin. Due to the high reactivity, intermediate (Ⅷ) instantly undergoes further nucleophilic addition with a molecule of 1, 3-indandione (Ⅲ) to produce another imine intermediate (Ⅸ), which tautomerizes to yield (Ⅹ). Finally, the intramolecular cyclisation of (Ⅹ) results in the ultimate spiro compound (Ⅳ) via tautomerization of (Ⅺ). The most observable feature is the formation of a more essential intermediate (Ⅷ), because it is the key intermediate for the final product.
|
Download:
|
| Scheme 1. Proposed mechanism for the synthesis of Ⅳ. | |
3.2. Pharmacology
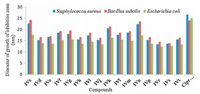
Sixteen compounds among all the synthesized were analyzed for their antibacterial and antifungal activity. All the compounds showed variable antibacterial activity against the Gram-positive bacteria namely (Staphylococcus aureus and Bacillus subtili) and Gram-negative bacteria (Escherichia coli) but no activity against Gram-negative (Pseudomonas aeruginosa) bacteria. The com-pounds showed good antifungal activity against yeast (Candida albicans and Saccharomyces cerevisiae). Positive controls produced significantly sized inhibition zones against the tested bacteria and fungi. However, negative control produced no inhibitory effect against any of the test organisms as shown in Table 3. Graphical representation of diameter of growth of inhibition (mm) of spiro[indolo-3, 100-indeno[1, 2-b]quinolin]-2, 4, 110-triones (Ⅳ) against bacteria and yeast strains is shown in Figs. 3 and 4.
|
|
Table 3 Antimicrobial activity of spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones (Ⅳ) through agar well diffusion method. |
|
Download:
|
| Figure 3. Graphical representation of diameter of growth of inhibition (mm) of spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones against bacteria strains. | |

|
Download:
|
| Figure 4. Graphical representation of diameter of growth of inhibition (mm) of spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones derivatives against yeast strains. | |
On the basis of maximum inhibitory activity shown against Gram positive bacteria, two compounds IVc and IVn were found to be most effective against B. subtilis and S. aureus with zone of inhibition of 22 mm and 24 mm and zone of inhibition of 17 mm against Gram negative bacteria. However in case of yeast, three compounds IVc, IVk and IVn were found to be best against C. albicans and S. cerevisiae with zone of inhibition of 15 mm and 16 mm, respectively (Table 3, Figs. 3 and 4).
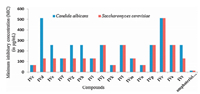
The minimum inhibitory concentration (MIC) of tested chemical compounds ranged between 8 and 512 μg/mL against bacteria and ranged between 64 and 512 μg/mL against yeast. Compounds IVc and IVn exhibited the lowest MIC of 16 μg/mL against S. aureus, compound IVc exhibited lowest MIC of 8 μg/mL against B. subtilis, and compound IVc was found to be best against E. coli with lowest MIC of 64 μg/mL. Three compounds namely IVc, IVk and IVn were found to be best against C. albicans and S. cerevisiae with lowest MIC of 64 μg/mL (Table 4). Among all the tested chemical compounds, two compounds namely IVc and IVn were found to be best in inhibiting the growth of bacteria and yeast. Graphical representationofMIC (in μg/mL) ofspiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones (IV) against bacteria and yeast strains is shown in Figs. 5 and 6.

|
Download:
|
| Figure 5. Graphical representation of Minimum inhibitory concentration (MIC) (in μg/mL) of spiro [indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones derivatives against bacteria strains. | |
|
Download:
|
| Figure 6. Graphical representation of Minimum inhibitory concentration (MIC) (in mg/mL) of spiro [indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'triones derivatives against yeast strains. | |
|
|
Table 4 Minimum inhibitory concentration (MIC) (in μg/mL) of spiro[indolo-3, 100-indeno[1, 2-b]quinolin]-2, 4, 11'-triones by using modified agar well diffusion method. |
4. Conclusion
In conclusion, we have reported a facile synthesis of spiro[indolo-3, 100-indeno[1, 2-b]quinolin]-2, 4, 110-triones in high yields using CAN in ethanol-water (1:1) under reflux in high yields. The antibacterial and antifungal activity of these compounds has also been reported. These synthesized compounds were evaluated for their antimicrobial activities, some of them have shown good antimicrobial and antifungal activities.
AcknowledgmentK.M. thanks Dyal Singh College, DU, for the grant of Teacher Fellowship and S.K. thanks CSIR, New Delhi, India for JRF and SRF.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.025.
| [1] | M. Grare, M. Mourer, S. Fontanay, In vitro activity of para-guanidinoethylcalix[4] arene against susceptible and antibiotic-resistant Gram-negative and Gram-positive bacteria.. J. Antimicrob. Chemother. (2007) 575–581. |
| [2] |
(a) K.C. Joshi, A. Dandia, S. Baweja, A. Joshi, Studies in spiroheterocycles. Part XVâ…¡. Synthesis of novel fluorine containing spiro[indole-pyranobenzopyran] and spiro[indenopyran-indole] derivatives, J. Heterocycl. Chem. 26(1989) 1097-1099; (b) G. Jones, W.J. Rae, Knoevenagel condensation products from some cyclic ketones:structure and stereochemistry, Tetrahedron 22(1966) 3021-3026. |
| [3] |
(a) A.H. Abdel-Rahman, E.M. Keshk, M.A. Hanna, S.M. El-Bady, Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents, Bioorg. Med. Chem. 12(2004) 2483-2488; (b) A. Dandia, R. Singh, S. Khaturia, et al., Efficient microwave enhanced regioselective synthesis of a series of benzimidazolyl/triazolyl spiro[indole-thiazolidinones] as potent antifungal agents and crystal structure of spiro[3H-indole-3, 2'-thiazolidine]-3'(1, 2, 4-triazol-3-yl)-2, 4'(1H)-dione, Bioorg. Med. Chem. 14(2006) 2409-2417. |
| [4] | V. Glover, J.M. Halket, P.J. Watkins, Isatin:identity with the purified endogenous monoamine oxidase inhibitor tribulin.. J. Neurochem. (1998) 656–659. |
| [5] | R. Kumar, R.C. Bansal, A. Mahmood, Isatin, an inhibitor of acetylcholinesterase activity in rat brain.. Biog. Amines 9 (1993) 281–289. |
| [6] | A.E. Medvedev, A. Clow, M. Sandler, V. Glover, Isatin:a link between natriuretic peptides and monoamines? Biochem. Pharmacol. 52(1996) 385-391. |
| [7] |
(a) S.K. Bhattacharya, V. Glover, I. McIntyre, G. Oxenkrug, M. Sandler, Stress causes an increase in endogenous monoamine oxidase inhibitor (tribulin) in rat brain, Neurosci. Lett. 92(1988) 218-221; (b) S.K. Bhattacharya, S.K. Mitra, S.B. Acharya, Anxiogenic activity of isatin, a putative biological factor, in rodents, J. Psychopharmacol. 5(1991) 202-206. |
| [8] | B.H. Rotstein, S. Zaretsky, V. Rai, A.K. Yudin, Small heterocycles in multicomponent reactions.. Chem. Rev. 114 (2014) 8323–8359. DOI:10.1021/cr400615v |
| [9] |
(a) Y.O. Teng, H.Y. Zhao, J. Wang, et al., Synthesis and anti-cancer activity evaluation of 5-(2-carboxyethenyl)-isatin derivatives, Eur. J. Med. Chem. 112(2016) 145-156; (b) X.M. Zhang, H. Guo, Z.S. Li, et al., Synthesis and evaluation of isatin-β-thiosemicarbazones as novel agents against antibiotic-resistant Gram-positive bacterial species, Eur. J. Med. Chem. 101(2015) 419-430; (c) P. Sharma, K.R. Senwar, M.K. Jeengar, et al., H2O-mediated isatin spiro-epoxide ring opening with NaCN:synthesis of novel 3-tetrazolylmethyl-3-hydroxy-oxindole hybrids and their anticancer evaluation, Eur. J. Med. Chem. 104(2015) 11-24. |
| [10] | A. Mondal, M. Brown, C. Mukhopadhyay, Multicomponent, one-pot and expeditious synthesis of highly substituted new spiro[indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones under micellar catalytic effect of CTAB in water.. RSC Adv. 4 (2014) 36890–36895. DOI:10.1039/C4RA04918G |
| [11] | S. Kumari, M. Rajeswari, J.M. Khurana, La (OTf)3-catalyzed, three-component synthesis of spiro[Indolo-3, 10'-indeno[1, 2-b]quinolin]-2, 4, 11'-triones in PEG-400 under conventional heating and ultrasonic irradiation.. Synth. Commun. 46 (2016) 387–394. DOI:10.1080/00397911.2016.1141427 |
| [12] |
(a) G. Khanna, K. Aggarwal, J.M. Khurana, An efficient and confluent approach for the synthesis of novel 3, 4-dihydro-2H-naphtho[2, 3-e][1, 3] oxazine-5, 10-dione derivatives by a three component reaction in ionic liquid, RSC Adv. 5(2015) 46448-46454; (b) P. Saluja, J.M. Khurana, C. Sharma, K.R. Aneja, An efficient and convenient approach for the synthesis of novel pyrazolo[12-a]triazole-triones and evaluation of their antimicrobial activities, Aust. J. Chem. 67(2014) 867-874; (c) H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Ultrasound promoted one pot synthesis of novel fluorescent triazolyl spirocyclic oxindoles using DBU based task specific ionic liquids and their antimicrobial activity, Eur. J. Med. Chem. 77(2014) 145-154; (d) M. Rajeswari, P. Saluja, J.M. Khurana, A facile and green approach for the synthesis of spiro[naphthalene-2, 50-pyrimidine]-4-carbonitrile via a one-pot three-component condensation reaction using DBU as a catalyst, RSC Adv. 6(2016) 1307-1312; (e) M. Rajeswari, J. Sindhu, H. Singh, J.M. Khurana, An efficient, green synthesis of novel regioselective and stereoselective indan-1, 3-dione grafted spirooxindolopyrrolizidine linked 1, 2, 3-triazoles via a one-pot five-component condensation using PEG-400, RSC Adv. 5(2015) 39686-39691. |
| [13] |
(a) C. Tietcheu, C. Garcia, D. Gardette, D. Dugat, J.C. Gramain, Efficient photochemical synthesis of tricyclic keto-indoles, J. Heterocycl. Chem. 39(2002) 965-973; (b) U.S. Sorensen, P. Villar, E. Helv, Synthesis of cyclopenta[b]indol-1-ones and carbazol-4-ones from N-(2-halophenyl)-substituted enaminones by intramolecular Heck reaction, Helvet. Chim. Acta 87(2004) 82-89; (c) F. Epifano, S. Genovese, M. Curini, Ytterbium triflate catalyzed synthesis of β-enaminones, Tetrahedron Lett. 48(2007) 2717-2720. |
| [14] | K.R. Aneja, C. Sharma, R. Joshi, In vitro efficacy of amaltas (Cassia fistula L.) against the pathogens causing otitis externa, J. Microbiol. 4(2011) 175-183. |
 2017, Vol. 28
2017, Vol. 28