Ionic liquids (ILs) are also known as room temperature ionic liquids, generally have a bulky volume, the asymmetric structure of the organic cation and relatively small volume of its inorganic or organic anion. They are salt compounds that are liquid at or near room temperature. Nowadays, ionic liquids are widely used in organic synthesis, catalysis, electrochemistry, analytical chemistry and many other fields [1-4]. Therefore, the development of ionic liquid detection methods is imperative. In 2011, the analysis of ionic liquid is reviewed by Paszkiewicz et al. [5]. At present, the analysis of ionic liquid cations mainly focuses on imidazolium [6-10] and pyridinium [11, 12] cations with ultraviolet absorption, and the analysis of the ionic liquid cations (piperidinium, pyrrolidinium and morpholinium) without ultraviolet absorption is relatively scarce [13-15]. The simultaneous analysis of piperidinium and pyrrolidinium cations has not been reported.
The aim of this work is to develop a method capable of rapid simultaneous separation and determination of piperidinium and pyrrolidinium ionic liquid cations by ion-pair chromatography. The mobile phase, column temperature, the effect of flow rate on retention of the cations were evaluated and the retention rules of the cations were proposed. A method was developed for the simultaneous analysis of three piperidinium and three pyrrolidinium cations by monolithic column ion-pair chromatography with direct conductivity detection, and the method was successfully applied to the analysis of the ionic liquid samples synthesized in our laboratory.
2. ExperimentalThe ILs (99% purity) studied were N-methyl-N-ethyl piperidinium bromide ([MEPi][Br]), N-methyl-N-propyl piperidinium bromide ([MPPi][Br]), N-methyl-N-butyl piperidinium bromide ([MBPi][Br]), N-methyl-N-ethyl pyrrolidinium bromide ([MEPy][Br]), N-methylN-propyl pyrrolidinium bromide ([MPPy][Br]), N-methyl-N-butyl pyrrolidinium bromide ([MBPy][Br]), and were supplied by Shanghai Cheng Jie Chemical Co., Ltd. (China). Sodium heptanesulfonate and sodium pentanesulfonate (Chromatographic purity) were supplied by Tianjin Fine Chemical Industry Research Institute (China). Acetonitrile (Chromatographic purity) was obtained from China Dimma Science and Technology Co[5_TD$DIF]., Ltd. (China).
Preparation and treatment of mobile phase: [6_TD$DIF]According to the experimental requirements, sodium heptanesulfonate or sodium pentanesulfonate was prepared in ultrapure water to produce a solution of accurate concentration and acetonitrile was then added. The mobile phase was filtered by 0.22 μm filter membrane and was degassed 15 min by vacuum degassing.
Preparation of standard solution: [7_TD$DIF]The standard solution of [MEPi][Br], [MPPi][Br], [MBPi][Br], [MEPy][Br], [MPPy][Br] and [MBPy][Br] was accurately weighed and diluted with ultrapure water until 1 g/L concentration of standard was acquired. The solutions were stored in a refrigerator, and diluted to the required concentration for the experiments.
Preparation of synthetic sample solution: 0.1-0.2 g of four ionic liquids synthesized, namely N-methyl-N-propyl pyrrolidinium bromide, N-methyl-N-ethyl piperidinium bromide, N-methyl-N-propyl piperidinium bromide, N-methyl-N-butyl pyrrolidinium bromide accurately was diluted to 100 mL as a concentrated solution. 0.75 mL of N-methyl-N-propyl pyrrolidinium bromide and N-methyl-N-ethyl piperidinium bromide, 1.0 mL of N-methylN-propyl piperidinium bromide, 1.5 mL of N-methyl-N-butyl pyrrolidinium bromide were diluted to 25 mL, filtered by 0.22 μm filter membrane and analyzed under the selected chromatographic conditions.
An LC-20A high performance liquid chromatograph (Shimadzu Corporation, Japan) was used, which consisted of a Model LP-20ADsp liquid delivery pump, a conductivity detector Model CDD-10Avp, an autosample injector Model SIL-20A, a Model CTO-20AC column oven and a system controller Model SCL-10Avp. All separations were performed on a Chromolith Speed ROD RP-18e column (4.6 mm × 50 mm, Merck Company, German). The suitable mobile phase was made of 0.5 mmol/L sodium heptanesulfonate and 5% acetonitrile (v/v). The flow rate was set at 3.0 mL/min. Column temperature was 30 ℃. The injection volume was 20 μL. Direct conductivity detection was employed. Shimadzu Solution Ver LC 1.1 chromatography workstation was used for data processing.
3. Results and discussionExperimental investigation was conducted on sodium heptanesulfonate and sodium pentanesulfonate, the two ion-pair reagent that have relative large hydrophobic difference. The study was also conduct to determine the effect of ion-pair reagent concentration on the ionic liquid cations’ retention and separation. The column temperature was set on 30 ℃. The flow rate was set at 3.0 mL/min. Using 0.5 mmol/L sodium heptanesulfonate-5% acetonitrile (v/v) and 0.5 mmol/L sodium pentanesulfonate-5% acetonitrile (v/v) as mobile phase, the effects of two mobile phases on retention and separation of six cations (N-methyl-N-ethyl piperidinium [MEPi]+, N-methyl-N-propyl piperidinium [MPPi]+, N-methyl-N-butyl piperidinium [MBPi]+, N-methyl-N-ethyl pyrrolidinium [MEPy]+, N-methyl-N-propyl pyrrolidinium [MPPy]+, N-methyl-N-butyl pyrrolidinium [MBPy]+) were investigated. The results show that when using a lesshydrophobic ion-pair reagent, sodium pentanesulfonate, the retention of six cations is weak. The short chain ILs, [MEPy]+, [MPPy]+ and [MEPi]+ were not well separated. However, the use of a larger hydrophobic ion-pair reagent, sodium heptanesulfonate, the result shows that the cations were relatively well separated. Therefore, sodium heptanesulfonate was chosen as the ion-pair reagent.
By maintaining a constant flow rate of mobile phase and column temperature, and varying 0.3 mmol/L, 0.5 mmol/L, 1.0 mmol/L, 1.5 mmol/L and 2.0 mmol/L sodium heptanesulfonate-5% acetonitrile (v/v) as mobile phase, the retention factors of six cations were investigated. The results show that the retention factors of six cations increased to different degrees with the increase of the concentration of sodium heptanesulfonate. When the concentration of sodium heptanesulfonate is lower than 0.5 mmol/L, the [MEPy]+ and [MEPi]+ peaks, due to their short retention time, interfered with the system peak. When the concentration of sodium heptanesulfonate is higher than 0.5 mmol/L, retention of the cations was too long and was not supportive to the further separation in which ion-pair reagents caused certain pollution on the column and peak shape variation. Therefore, 0.5 mmol/L of sodium heptanesulfonate was used in the experiment. At this concentration, the chromatographic peaks of cations were better, and column pollution was weak.
The separation process of the cations may involve the following two mechanisms: hydrophobic interactions as ion-pair reagent concentration increases in the mobile phase, the capability of the ion-pair reagent and analyte cations to form neutral ion increased, resulting in enhanced hydrophobic interactions between the neutral ion-pair and the stationary phase, and eventually the retention of cation increased. The other mechanism could be ion exchange. As the ion-pair reagent concentration increased, the adsorption of ion-pair reagent on the surface of the column increased, thus resulting in larger ion exchange capacity, and enhanced cation retention.
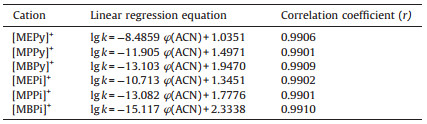
The first choice of organic modifier in the mobile phase used in this study is acetonitrile. The main reason is that the mixture of acetonitrile and water is of low viscosity, resulting in fewer bubbles. At a column temperature of 30 ℃, a mobile phase flow rate of 3.0 mL/min, with 0.5 mmol/L sodium heptanesulfonate-3%, 4%, 5%, 6% and 7% acetonitrile (v/v) as mobile phase, the effect of acetonitrile content on the retention of six cations was investigated. In liquid chromatography, using acetonitrile as mobile phase, the relationship between the retention factor of analytes and the concentration of acetonitrile is as follows, lg k=c φ(ACN) + d [6] (k is the retention factor, φ(ACN) is the volume fraction of acetonitrile). By mapping the lg k and φ(ACN) relationship, the linear regression data of the resulting curve are shown in Table 1. The data in Table 1, show that each cation has a good linear relationship, the slope is negative, the retention factor is shortened with the increased acetonitrile content in mobile phase. When acetonitrile content was less than 5%, the separation of [MEPy]+[8_TD$DIF], [MEPi]+, [MPPy]+ and [MPPi]+ was better, however, [MBPy]+ and [MBPi]+ retention time was too long and the peak shape was poor. When the acetonitrile content was more than 5%, the peak shape of [MBPy]+ and [MBPi]+ improved, but [MEPy]+ and [MEPi]+ peaks interfered with the system peak. In order to reach the goal of simultaneous separation of six cations, 5% acetonitrile was chosen. If only the analysis of [MBPy]+ and [MBPi]+ was needed, the acetonitrile content of mobile phase can be increased. The above two mechanisms showed that with the increase of acetonitrile volume fraction in mobile phase, the hydrophobicity of the mobile phase increased, this led to the combination of cations and ion-pair reagent forming the neutral species in a hydrophobic environment and the decrease of analyte retention factor. Because of the different polarity of cations, the reduction range of each ion was also different. In addition, the addition of acetonitrile reduced the adsorption capacity of ion-pair reagent on the column surface, and reduced the exchange capacity of column, thus the retention factor of each cation is naturally reduced.
|
|
Table 1 Linear regression equations of the lg k vs. φ(ACN) for cations. |
Using 0.5 mmol/L sodium heptanesulfonate-5% acetonitrile (v/v) as mobile phase, the flow rate of 3.0 mL/min, the effects of temperature (25 ℃, 30 ℃, 35 ℃, 40 ℃) on the retention of the cations were investigated. The results showed that with the increase of column temperature, the retention time of the six cations was slightly shorter, but the overall effect was negligible. The Fant Hoff curves of cation were obtained in the investigated temperature range as ln k=-ΔH/(RT) + ΔS/R + ln φ [16, 17] (k is the retention factor, T is the thermodynamic temperature, ΔH and -ΔS are the changes of ion exchange reaction free enthalpy and free entropy, R is the molar gas constant, φ is two phase volume ratio), see Table 2. According to the data of Table 2, the slope of the cation is positive, and the retention of the six cations is an exothermic process, that is, the retention of the cation decreases with the increase of temperature. By a comprehensive consideration, the selected column temperature is 30 ℃.
|
|
Table 2 Linear regression equations of ln k vs. 1/T for the cations. |
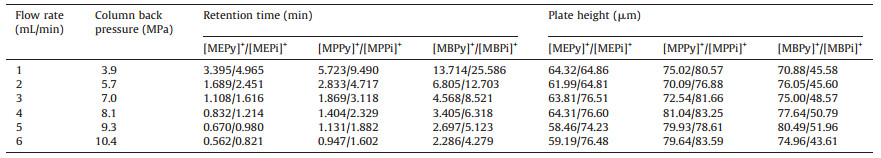
Under the above selected conditions, the flow rate was investigated between 1.0 mL/min and 6.0 mL/min. The effects of flow rate on retention time, column pressure and column efficiency are shown in Table 3. From Table 3, it was shown that with the increase of the flow rate, as predicted, the retention time of each ion is shortened, and the column pressure gradually increased, but the column efficiency has a minimal change. When the flow rate is greater than 3 mL/min, the noise level of the base line is high. Therefore, the flow rate of 3 mL/min was used as the best velocity of the experiment. Under these conditions, six cations can be well separated in 10 min.
|
|
Table 3 Effect of flow rate on retention time of cation, column back-pressure and column efficiency. |
There is a carbon number rule between carbon atom number and homologue retention factor, which follows lg k=anc + b [6] (k is the retention factor, nc is the homologue carbon atom number). Under the selected chromatographic conditions, with 0.5 mmol/L sodium heptanesulfonate-5% acetonitrile (v/v) as mobile phase, a flow rate of 3.0 mL/min, the column temperature 30 ℃, six cations are analyzed. Mapping the retention factor of cation with the carbon atom number of the cationic alkyl side chain, the carbon number rules of the cations were obtained as the follows: pyrrolidinium cationic homologue, lg k=0.0388nc -0.3703, r=0.9947; piperidinium cation homologue, lg k=0.027 nc -0.2805, r=0.9900. The equations showed that lg k and nc have a good linear relationship, indicating that under these conditions, retention factor of pyrrolidinium cations and piperidinium cations conformed with the carbon number rule.
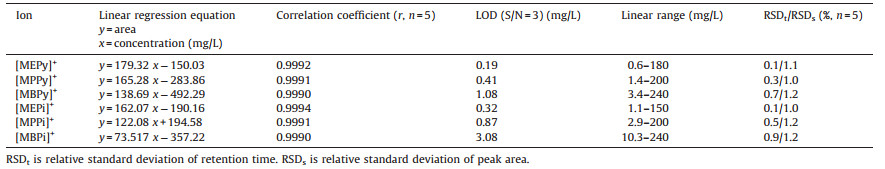
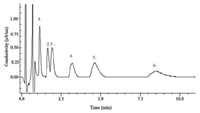
According to the chromatographic conditions described in the experimental section, five different concentrations of mixed standard solution consisting of six cations were determined. Linear regression equations of six cations were obtained from the relationship between the peak area integral value (y) and ionic concentration (x, mg/L). Detection limits were calculated with a three times of signal-to-noise ratio, and noise of the baseline was 0.002 mS/cm. The optimum chromatogram Fig. 1 and the repeatability of the method were obtained by five measurements of a standard mixture solution of [MEPy]+ (30 mg/L), [MPPy]+ (40 mg/L), [MBPy]+ (60 mg/L), [MEPi]+ (30 mg/L), [MPPi]+ (40 mg/L) and [MBPi]+ (60 mg/L). Separation of the cations was achieved within 10 min. The chromatographic peaks 4, 5, 6 have some trailing phenomenon in the figure. The main reason is that the chromatographic column was used for a long time, resulting to the decreaseofcolumnefficiency. Thelongerretention timesofionsalso have more prone to trailing. The data are listed in Table 4. As can be seen from Table 4, this method produced a good linear relationship, acceptable detection limit and good precision.
|
|
Table 4 Linear regression equation, limits of detection (LOD) and relative standard deviation (RSD) of cations. |
|
Download:
|
| Figure 1. Chromatogram of a standard mixture of ionic liquid cations. Chromatographic conditions: Chromolith Speed ROD RP-18e column; mobile phase, 0.5 mmol/L sodium heptanesulfonate-5% acetonitrile (v/v). flow rate, 3.0 mL/min; column temperature, 30 ℃; inject volume, 20 μL; detection, direct conductivity detection.Peaks (mg/L):(1) [MEPy]+(30);(2)[MEPi]+(30);(3) [MPPy]+ (40); (4) [MPPi]+ (40); (5) [MBPy]+ (60); (6) [MBPi]+ (60). | |
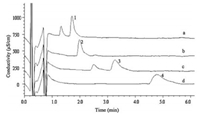
This method was applied to the determination of four ionic liquid samples synthesized by our chemistry lab, namely N-methyl-N-propyl pyrrolidinium bromide [MPPy][Br], N-methyl-N-butyl pyrrolidinium bromide [MBPy][Br], N-methyl-N-ethyl piperidinium bromide [MEPi][Br] and N-methyl-N-propyl piperidinium bromide [MPPi][Br]. The preparation of the ionic liquid sample solution is shown in the experimental section, and then using the best chromatographic conditions, the chromatogram in Fig. 2 was obtained. The recovery rate of the method was determined by the standard addition method, and the content of the cations in the ionic liquid samples and the recovery data are shown in Table 5. The data in Table 5 showed that the RSD were less than 3.4%. The cation content (%) in the ionic liquid sample was calculated using the mass ratio of the cation in the ionic liquid samples. The results showed that the method were accurate and reproducible, and met the requirements of quantitative analysis of the ionic liquid samples.
|
|
Table 5 Concentrations and recoveries of cations spiked in ionic liquid samples. |
|
Download:
|
| Figure 2. Chromatograms of ionic liquid samples. Ionic liquid samples: (a) N-methylN-ethyl piperidinium bromide ionic liquid sample; (b) N-methyl-N-propyl pyrrolidinium bromide ionic liquid sample; (c) N-methyl-N-propyl piperidinium bromide ionic liquid sample; (d) N-methyl-N-butyl pyrrolidinium bromide ionic liquid sample. Peaks: (1) [MEPi]+; (2) [MPPy]+; (3) [MPPi]+; (4) [MBPy]+. | |
4. Conclusion
A method of ion-pair chromatography with direct conductivity detection was developed on a silica-based monolithic column for the fast and simultaneous determination of piperidinium and pyrrolidinium ionic liquid cations. Sodium heptanesulfonate is more suitable for the separation and determination of the cations than sodium pentanesulfonate, and with the increase in ion-pair reagent concentrations, the retention time of cation increased. The higher content of acetonitrile and the higher flow rate shortened the retention time of cations. The retention process of piperidinium and pyrrolidinium cations is exothermic, and the retention of cationic homologues conforms to the rule of carbon number. This method has been successfully applied to determine two piperidinium cations and two pyrrolidinium cations in ionic liquid samples synthesized by our chemistry lab. The results were accurate and reproducible, and met the requirements of quantitative analysis of ionic liquid samples.
Acknowledgment This work was supported by the Natural Science Foundation of Heilongjiang Province (No. B201307).| [1] | M.L. Zhang, A.K. Mallik, M. Takafuji, H. Ihara, H.D. Qiu, Versatile ligands for highperformance liquid chromatography:an overview of ionic liquid-functionalized stationary phases. Anal. Chim. Acta 887 (2015) 1–16. DOI:10.1016/j.aca.2015.04.022 |
| [2] | Y.Q. Cai, G.H. Song, D.N. Liang, Desulfurization using the 1, 2-dimethylimidazolium ionic liquid as an adsorbent. Chin. Chem. Lett. 26 (2015) 317–319. DOI:10.1016/j.cclet.2014.11.027 |
| [3] | X.X. Qin, Z.L. Cheng, Application of ionic liquids as a catalyst in the synthesis of polyvinyl butyral (PVB) polymer. Chin. Chem. Lett. 27 (2016) 145–148. DOI:10.1016/j.cclet.2015.07.012 |
| [4] | Y.J. Ma, C. Guan, Y.J. Dong, H. Yu, High-performance liquid chromatography utilization of ionic liquids as mobile phase additives for separation and determination of the isomers of amino benzoic acids. Chin. Chem. Lett. 27 (2016) 749–752. DOI:10.1016/j.cclet.2016.01.023 |
| [5] | M. Paszkiewicz, P. Stepnowski, How should ionic liquids be analyzed? Curr. Org. Chem. 15(2011) 1873-1887. |
| [6] | M.J. Ruiz, - Angel, A. Berthod, Reversed phase liquid chromatography of alkylimidazolium ionic liquids. J. Chromatogr. A 1113 (2006) 101–108. DOI:10.1016/j.chroma.2006.01.124 |
| [7] | S. Studzi, ń ska, B. Buszewski, Study of retention mechanism of imidazolium-based ionic liquids in HPLC. J. Sep. Sci. 33 (2010) 1264–1273. |
| [8] | X. Huang, H. Yu, Y.J. Dong, Rapid and simultaneous determination of imidazolium and pyridinium ionic liquid cations by ion-pair chromatography using a monolithic column. Chin. Chem. Lett. 23 (2012) 843–846. DOI:10.1016/j.cclet.2012.04.029 |
| [9] | C.X. Lu, Z.G. Tang, C.B. Liu, Determination of ionic liquid cations in soil samples by ultrasound-assisted solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Methods 7 (2015) 5924–5933. DOI:10.1039/C5AY00750J |
| [10] | C.A. Hawkins, A. Rud, M.L. Guthrie, M.L. Dietz, Rapid quantification of imidazolium-based ionic liquids by hydrophilic interaction liquid chromatography:methodology and an investigation of the retention mechanisms. J. Chromatogr. A 1400 (2015) 54–64. DOI:10.1016/j.chroma.2015.04.047 |
| [11] | Q. Chen, H. Yu, J.F. Wang, Determination of pyridinium ionic liquid cations by ion chromatography with direct conductivity detection. J. Liq. Chromatogr. Relat. Technol. 35 (2012) 1184–1193. DOI:10.1080/10826076.2011.619028 |
| [12] | F. Onink, W. Meindersma, B. Burghoff, Ion chromatography as a novel method to quantify the solubility of pyridinium ionic liquids in organic solvents. J. Chromatogr. Sci. 53 (2015) 8–15. DOI:10.1093/chromsci/bmu001 |
| [13] | H. Yu, Y.M. Sun, C.M. Zou, Imidazolium ionic liquid as the background ultraviolet absorption reagent for determination of morpholinium cations by high performance liquid chromatography-indirect ultraviolet detection. Chin. Chem. Lett. 25 (2014) 1371–1374. DOI:10.1016/j.cclet.2014.05.041 |
| [14] | C. Guan, H. Yu, Hydrophilic interaction liquid chromatography with indirect ultraviolet detection for the separation and quantification of pyrrolidinium ionic liquid cations. Chin. Chem. Lett. 26 (2015) 1371–1375. DOI:10.1016/j.cclet.2015.08.004 |
| [15] | Y. Zhang, H. Yu, M.Y. Wang, Determination of pyrrolidinium ionic liquid cations by ion chromatography-indirect ultraviolet detection with imidazolium ionic liquids as both an ultraviolet absorption reagent and an eluting agent. Anal. Methods 7 (2015) 5654–5660. DOI:10.1039/C5AY01211B |
| [16] | H. Yu, S.F. Mou, Effect of temperature on the retention of amino acids and carbohydrates in high-performance anion-exchange chromatography. J. Chromatogr. A 1118 (2006) 118–124. DOI:10.1016/j.chroma.2005.12.051 |
| [17] | H. Yu, R.S. Li, Effect of column temperature on the retention of inorganic anions and organic acids in non-suppressed anion-exchange IC. Chromatographia 68 (2008) 611–616. DOI:10.1365/s10337-008-0774-4 |
 2017, Vol. 28
2017, Vol. 28