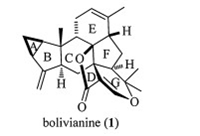
Bolivianine is a structurally intriguing sesterterpenoid as shown in Fig. 1, isolated from Hedyosmum angustifolium (Chloranthaceae) by the Jullian group in 2007 [1]. Although no bioactivity of this sesterterpenoid was reported, we were attracted by its structural complexity and synthetic challenge. In 2013, our group reported the first and bioinspired asymmetric total synthesis of bolivianine with commercially available (+)-verbenone as the starting material [2]. Led by our biogenetic hypothesis, this total synthesis features efficient assembly of a 3/5/6 architecture through intramolecular cyclopropanation of an allylic metal carbene intermediate [3], and an ambitious Diels-Alder/intramolecular hetero-Diels-Alder (DA/IMHDA) cascade to install the EFG tricycle of bolivianine in one pot [4].
|
Download:
|
| Figure 1. Molecular structures of bolivianine. | |
In our previous bioinspired total synthesis of bolivianine as illustrated in Scheme 1 [2, 4], compound 3 was prepared from (+)-verbenone in seven steps. Aldol reaction with compound 4 and subsequent acidic treatment transformed 3 to the furan 5 in one pot. DIBAL-H reduction and silyl protection afforded compound 6, which was elaborated after oxidative dearomatization and silyl deprotection to produce onoseriolide 7, a natural sesquiterpenoid [5]. Final IBX oxidation and biomimetic DA/IMHDA cascade with the monoterpene 9 [6] furnished bolivianine in 14 steps. Actually, we were unsatisfied with the synthetic procedure from compound 3 to the target molecule since it is not concise enough. Herein we report our efforts on the promotion of our previous total synthesis.

|
Download:
|
| Scheme 1. Our previously published bioinspired total synthesis of bolivianine (1). | |
2. Experimental
All reactions were carried out under an argon atmosphere with dry solvents under anhydrous conditions, unless otherwise noted. Dichloromethane (DCM), diisopropylamine (DIPA), 1, 8-diazabicyclo[5.4.0]undec-7-ene (DBU) and toluene were distilled from calcium hydride under argon. Tetrahydrofuran was distilled from sodium-benzophenone. All the chemicals were purchased commercially and used without further purification, unless otherwise stated. Flash chromatography was performed using silica gel (200-400 mesh). Reactions were monitored by thin layer chromatography (TLC). Visualization was achieved under a UV lamp (254 nm and 365 nm), I2 and by developing the plates with p-anisaldehyde or phosphomolybdic acid. NMR were recorded on Bruker DRX-400 MHz NMR spectrometer with TMS as the internal standard and were calibrated using residual undeuterated solvent as an internal reference (CDCl3: 1H NMR=7.26, 13C NMR=77.16; C6D6: 1H NMR=7.28, 13C NMR=127.82). Coupling constants (J) are reported in Hertz (Hz). Optical rotations were measured at the sodium D line with a 100 mm path length cell, and are reported as follows: [α]DT, concentration (g/100 mL), and solvent. High resolution Mass spectra (HRMS) were recorded by using FTMS-7 spectrometers. Infrared spectra (IR) were recorded on a NEXUS 670 Fourier transform infrared spectrophotometer and are reported in wavenumbers (cm-1).
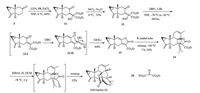
Preparation of compound 11 (Scheme 2): To a stirred solution of lithium diisopropylamide (LDA), prepared from diisopropylamine (DIPA) (534.3 mg, 0.75 mL, 5.28 mmol) and n-BuLi (2.5 mol/L in hexane, 2.11 mL, 5.28 mmol) in THF (20 mL), was added a solution of 3 (374.3 mg, 2.11 mmol) in THF (5 mL) at 0 ℃. After the resulting mixture was stirred for 30 min at the same temperature, the mixture of diethyl ketomalonate 10 (919.5 mg, 0.81 mL, 5.28 mmol) and ZnCl2 (719.6 mg, 5.28 mmol) in THF (15 mL) was added dropwise to the above solution at 0 ℃. After stirring for another 1 h at 0 ℃, the resulting mixture was quenched with saturated NH4Cl solution (20 mL). The layers were separeted and the aqueous layer was extracted with EtOAc (2×30 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (6:1 petroleum ether/EtOAc) to give compound 11 (488.0 mg, 66%) as a colorless oil.
|
Download:
|
| Scheme 2. The promoted total synthesis of bolivianine. | |
Preparation of compound 12: To a stirred solution of 11 (112.5 mg, 0.32 mmol) in acetic anhydride (0.3 mL) was added InCl3 (7.1 mg, 0.03 mmol) in acetic anhydride (0.7 mL) slowly at 0 ℃. The resulting mixture was stirred for 15 min at this temperature. The reaction was quenched with saturated NaHCO3 solution (60 mL), and the resulting mixture was stirred over night at 0 ℃. The layers were seperated and the auqeous layer was extracted with EtOAc (2 × 30 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (4:1 petroleum ether/EtOAc) to give compound 12 (85.4 mg, 72%) as a light yellow oil.
Preparation of compound 13: To a stirred solution of lithium bromide (208.5 mg, 2.40 mmol) in THF (10 mL) was added 12 (85.4 mg, 0.22 mmol) in THF (5 mL) dropwise at -78 ℃. After stirring for 5 min, a solution of DBU (365.4 mg, 0.36 mL, 2.40 mmol) in THF (5 mL) was added slowly to this solution and the reaction mixture was stirred at -78 ℃ for 4 h. Then the mixture was warmed to -20 ℃ slowly and the mixture was stirred overnight. The reaction was quenched with saturated NH4Cl solution (10 mL). The layers were seperated and the aqueous layer was extracted with EtOAc (2 × 10 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (4:1 petroleum ether/EtOAc) to give compound 13 (40.2 mg, 64%) as a light yellow oil. [α]D26 + 6.4 (c 0.09, CHCl3); IR (thin film): 3446, 2958, 2925, 2854, 1790, 1714, 1617, 1379, 1318, 1217, 1027, 798 cm-1; 1H NMR (400 MHz, CDCl3): δ 6.68 (s, 1H), 5.10 (bs, 1H), 4.85 (bs, 1H), 4.36 (q, 2H, J=7.2 Hz), 3.39 (dd, 1H, J=3.6, 18.8 Hz), 3.02 (dd, 1H, J=2.4, 13.6 Hz), 2.57 (dd, 1H, J=13.6, 18.4 Hz), 2.03-1.98 (m, 1H), 1.71 (td, 1H, J=4.0, 7.6 Hz), 1.38 (t, 3H, J=7.2 Hz), 0.96 (dd, 1H, J=1.6, 3.6 Hz), 0.95-0.92 (m, 1H), 0.83 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 165.3, 162.0, 161.6, 149.2, 148.6, 128.8, 115.6, 107.5, 61.6, 40.4, 29.8, 26.5, 24.1, 22.5, 22.0, 17.4, 14.4; HRMS (ESI): m/z calcd. for C17H18O4Na [M+Na]+ 309.1103, found 309.1105.
Preparation of compound 14: To a stirred solution of 13 (40.2 mg, 0.14 mmol) in toluene (1.5 mL) was added β-E-ocimene 9 (171.1 mg, 1.40 mmol) in toluene (1.3 mL). The reaction mixture was heated at 150 ℃ in a sealed-tube for 7 h. After evaporation of the solvent, the residue was purified by column chromatography on silica gel (6:1 petroleum ether/EtOAc) to give compound 14 (31.8 mg, 54%) as a light yellow oil. [α]26 D -47.1 (c 0.24, CHCl3); IR (thin film): 3406, 3079, 2972, 2922, 1773, 1712, 1639, 1409, 1331, 1070, 629 cm-1; 1H NMR (400 MHz, CDCl3): δ 5.75 (d, 1H, J=7.2 Hz), 5.04 (s, 1H), 5.00 (dd, 1H, J=6.4, 7.6 Hz), 4.79 (s, 1H), 4.39-4.30 (m, 2H), 3.19-3.08 (m, 2H), 2.91-2.82 (m, 1H), 2.52-2.47 (m, 1H), 2.40-2.30 (m, 2H), 2.03-1.96 (m, 2H), 1.84 (dd, 1H, J=4.0, 7.2 Hz), 1.77 (s, 3H), 1.66 (s, 3H), 1.65-1.61 (m, 2H), 1.52 (s, 3H), 1.37 (t, 3H, J=7.2 Hz), 0.83-0.78 (m, 1H), 0.71-0.68 (m, 1H), 0.57 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 186.4, 167.3, 161.8, 151.9, 141.2, 134.0, 121.8, 121.0, 119.9, 106.9, 94.8, 61.4, 57.7, 54.5, 51.1, 42.4, 28.5 (2C), 25.7, 24.5 (2C), 24.4, 23.7, 22.9, 18.0, 16.4, 14.3; HRMS (ESI): m/z calcd. for C27H34O4Na [M+Na]+ 445.2349, found 445.2353.
Preparation of bolivianine (1): To a stirred solution of compound 14 (24.0 mg, 0.0568 mmol) in DCM (2.0 mL) was added DIBAL-H (1.0 M in hexane, 62.5 mL, 0.0625 mmol) at -78 ℃. After stirring at this temperature for 1 h, MeOH (1.0 mL) was added to quench the reaction. The mixture was warmed to room temperature and aqueous saturated Rochelle salt solution (2.5 mL) was added to the mixture. The mixture was stirred vigorously for 3 h. Then the mixture was extracted with EtOAc (3 × 10 mL), and the combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (20: 1 petroleum ether/ EtOAc) to provide bolivianine 1 (11.2 mg, 52%) as a white solid. [α]D 26 -52.0 (c 0.1, CHCl3), {Lit. [1] [α]25 D -50.0 (c 0.2, CHCl3)}; IR (thin film): 2926, 2851, 1753, 1644, 1449, 1374, 1227, 877 cm-1; 1H NMR (400 MHz, C6D6): δ 7.39 (s, 1H), 5.64 (m, 1H), 5.18 (bs, 1H), 4.74 (bs, 1H), 2.63 (dd, 1H, J=8.0, 12.0 Hz), 2.56 (bdd, 1H, J=2.4, 12.8 Hz), 2.32-2.29 (m, 1H), 2.28-2.24 (m, 1H), 2.07 (ddd, 1H, J=6.8, 12.0, 14.0 Hz), 1.94 (dd, 1H, J=2.0, 14.0 Hz), 1.94 (m, 1H), 1.78-1.73 (m, 1H), 1.69 (dd, 1H, J=6.8, 10.8 Hz), 1.63 (bt, 3H, J=2.0 Hz), 1.49-1.44 (m, 1H), 1.36 (dd, 1H, J=13.2, 14.0 Hz), 1.17 (s, 3H), 1.13-1.08 (m, 1H), 1.02 (s, 3H), 1.00 (s, 3H), 0.91 (dd, 1H, J=3.2, 8.4 Hz, ), 0.70 (td, 1H, J=5.2, 8.8 Hz); 13C NMR (100 MHz, C6D6): δ 168.5, 152.9, 149.4, 140.0, 120.5, 110.3, 105.4, 96.9, 78.5, 52.5, 52.1, 51.6, 46.6, 44.3, 39.8, 35.4, 27.3, 27.2, 26.2, 26.1, 23.6, 23.4, 22.6, 20.5, 16.3; HRMS (ESI): m/z calcd. for C25H31O3 [M+H]+ 379.2273, found 379.2271.
3. Results and discussionCompound 3 was prepared according to our established synthetic procedure [2], but the yield of the intramolecular cyclopropanation was promoted from the original 65% to the current 81% [3]. This compound was deprotonated to go through aldol reaction with commercially available compound 10 [4, 7] to afford compound 11 as an inseparable mixture of diastereomers. Direct transformation from 11 to 13 failed in spite of numerous trials. Thus the tertiary alcohol in 11 was acetylated [8] to produce compound 12. Then compound 12 was converted into compound 13 in the presence of both DBU and lithium bromide. In this process, compound 12 was supposed to afford the intermediate 12-Ⅰ at first through elimination of one molecule of acetic acid. Activated by LiBr as Lewis acid, the ester in 12-Ⅱ can be attacked by the enolate generated in situ in the presence of DBU, to form compound 13. Enlightened by the facile reactivity between compound 8 and compound 9 (Scheme 1), we mixed 13 and 9 in toluene in a sealed tube. Thermal Diels-Alder cycloaddition proved feasible at 150 ℃ and thus delivered compound 14 in 54% yield. By following our previous findings [4], compound 14 was transformed to bolivianine (1) in 52% yield through selective reduction of the ethyl ester with DIBAL-H, followed by spontaneous intramolecular hetero-Diels-Alder reaction upon workup with Rochelle salt. Accordingly, at present, we can accomplish total synthesis of bolivianine (1) in only twelve steps from (+)-verbenone, two steps shorter than our previously established bioinspired total synthesis with comparable overall yield.
4. ConclusionIn general, we completed an alternative total synthesis of bolivianine on the basis of our previously published work. At the current stage, we are able to achieve the target molecule through shorter synthetic sequence (12 steps in total), which would benefit synthesis of analogues of bolivianine and isobolivianine and evaluation of their bioactivities.
AcknowledgmentWe appreciate the financial support from the National Natural Science Foundation of China (Nos. 21322205, 21321061) and the Ministry of Education of China (No. 20130181110022).
| [1] | L. Acebey, M. Sauvain, S. Beck, Bolivianine, a new sesterpene with an unusual skeleton from Hedyosmum angustifolium, and its isomer, isobolivianine. Org. Lett. 9 (2007) 4693–4696. DOI:10.1021/ol7015725 |
| [2] | C. Yuan, B. Du, L. Yang, B. Liu, Bioinspired total synthesis of bolivianine:a DielsAlder/intramolecular hetero-Diels-Alder cascade approach. J. Am. Chem. Soc. 135 (2013) 9291–9294. DOI:10.1021/ja4040335 |
| [3] | Y. Yang, J. Li, B. Du, An entry to vinylcyclopropane through palladiumcatalyzed intramolecular cyclopropanation of alkenes with unstabilized allylic tosylhydrazones. Chem. Commun. 51 (2015) 6179–6182. DOI:10.1039/C5CC00235D |
| [4] | B. Du, C. Yuan, T. Yu, Asymmetric total synthesis of onoseriolide, bolivianine, and isobolivianine. Chem. Eur. J. 20 (2014) 2613–2622. DOI:10.1002/chem.v20.9 |
| [5] |
(a) S.K.S. Amoah, F.L. Oliveira, M.W. Biavatti, et al., Sesquiterpene lactones from the leaves of Hedyosmum brasiliense (Chloranthaceae), Phytochemistry 87(2013) 126-132; (b) L. Acebey, V. Jullian, M. Sauvain, et al., Anti-Leishmanial Lindenane Sesquiterpenes from Hedyosmum angustifolium, Planta Med. 76(2010) 365-368; (c) A.P. Trentin, A.R.S. Santos, J.B. Calixto, Antinociception caused by the extract of Hedyosmum brasiliense and its active principle, the Sesquiterpene Lactone 13-Hydroxy-8, 9-dehydroshizukanolide, Planta Med. 65(1999) 517-521; (d) F. Bohlmann, C. Zdero, H. Robinson, et al., Onoseriolid, ein neues sesquiterpenlacton aus Onoseris albicans, Phytochemistry 19(1980) 689-691. |
| [6] | H. Cong, C.F. Becker, S.J. Elliott, Silver nanoparticle-catalyzed Diels-Alder cycloadditions of 2'-hydroxychalcones. J. Am. Chem. Soc. 132 (2010) 7514–7518. DOI:10.1021/ja102482b |
| [7] | A. Armstrong, T.J. Critchley, A.A. Mortlock, Synthesis studies on CP-225, 917 and CP-263, 114:concise synthesis of the bicycle core using an intramolecular Mukaiyama aldol reaction. J. Chem. Soc. Perkin Trans. 1 (2002) 1344–1350. |
| [8] |
(a) A.K. Chakraborti, L. Sharma, R. Gulhane, et al., Electrostatic catalysis by ionic aggregates:scope and limitations of Mg (ClO4)2 as acylation catalyst, Tetrahedron 59(2003) 7661-7668; (b) A.K. Chakraborti, R. Gulhane, Indium (Ⅲ) chloride as a new, highly efficient, and versatile catalyst for acylation of phenols, thiols, and amines, Tetrahedron Lett. 44(2003) 6749-6753; (c) M.S. Niasari, T. Khosousi, S. Hydarzadeh, Highly selective esterification of tertbutanol by acetic acid anhydride over alumina-supported InCl3, GaCl3, FeCl3, ZnCl2, CuCl2, NiCl2, CoCl2 and MnCl2 catalysts, J. Mol. Catal. A:Chem. 235(2005) 150-153. |
 2017, Vol. 28
2017, Vol. 28 


