b School of Chemistry, Biology and Material Science, East China University of Technology, Nanchang 330013, China
2, 3-Dihydroquinazolin-4(1H)-one derivatives acquired significance due to their broad range of potential biological and pharmacological activities, as well as their crucial role in the preparation of drug molecules and natural products. 2, 3-Dihydroquinazolinone derivatives act as important intermediates that can be easily oxidized to their quinazolin-4(3H)-one analogues, which also include important biologically active compounds. In recent years, the protocols for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones have been developed in different ways using iodine [1], succinimide-N-sulfonic acid [2], p-TSA [3], 2-morpho-linoethanesulfonic acid [4], ZrCl4 [5], NH4Cl [6], KAl (SO4)2 [7], silica sulfuric acid [8], sulfamic acid [9], nanocrystalline sulfated zirconia [10], N-propylsulfamic acid on magnetic nanoparticles [11], metalCNTs [12], β-cyclodextrin-SO3H [13], PEG-400 [14] and ionic liquids [15] as catalysts. These synthetic methodologies are useful to facilitate the synthesis of the desired compounds in many instances. However some of the synthetic strategies have certain limitations such as tedious processes, long reaction time, low yields of product, expensive moisture-sensitive catalysts, cumbersome preparation processes for the required catalyst, and liberating hazardous HF during recycling. Therefore, there has been increasing effort on the design and use of environmentally compatible catalysts for the preparation of 2, 3-dihydroquinazolin-4(1H)-one derivatives owing to the growing demand for the development of more sustainable and environmentally-friendly processes.
Enzymes have received great attention as sustainable, efficient, green and biodegradable catalysts for the synthesis of pharmaceutical, industrial and agricultural chemicals and intermediates. Among the promiscuous enzymes, hydrolases beyond doubt play an important role because of their high stability, availability, and broad range of substrates. Recently, several enzymes have been used in multiple types of organic reactions such as asymmetric aldol reactions [16], asymmetric Michael additions [17] and asymmetric Henryreactions[18].However, tothebestofourknowledge, enzymecatalyzed cyclocondensation reaction for the preparation of 2, 3-dihydroquinazolin-4(1H)-one derivatives has never been reported. Herein, we wish to report the first discovery that α-chymotrypsin could catalyze the reaction of 2-aminobenzamide with a series of aldehydes to afford the corresponding 2, 3-dihydroquinazolinones in ethanol (Scheme 1), and the yields up to 98% were achieved, which was obviously a novel environmentally benign synthetic method.

|
Download:
|
| Scheme 1. The α-chymotrypsin-catalyzed cyclocondensation of 2-aminobenzamide with aldehydes. | |
2. Experimental 2.1. General
Alkaline protease (200 U/mg enzyme activity) and Papain (800 U/mg enzyme activity) were purchased from Jiangsu Ruiyang Biotechnology Co., Ltd. (Jiangsu, PR China). Trypsin from bovin pancreas (≥2500 U/mg enzyme activity), α-chymotrypsin (800 U/mg enzyme activity) and albumin from bovine serum were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, PR China). Protease from Aspergillus saitoi (≥0.6 U/mg enzyme activity), proteinase from Aspergillus melleus (3.3 U/mg enzyme activity) and protease from Bacillus licheniformis (2.4 U/g enzyme activity) were purchased from Sigma-Aldrich Co., USA. All reagents were obtained from commercial suppliers and used without further purification except that 2-aminobenzamide was recrystallized. Melting points were obtained on a WRS-1B Digital Melting Point Apparatus. The NMR spectra were recorded on a Bruker 400 MHz instrument using DMSO-d6 as a solvent. Chemical shifts (δ) were expressed in ppm using tetramethylsilane (TMS) as an internal standard, and coupling constants (J) were reported in Hz.
2.2. General procedure for the synthesis of 2, 3-dihydroquinazilin-4(1H)-onesα-Chymotrypsin (2 mg/mL) was added to a solution of 2-aminobenzamide (1 mmol) and aldehyde (1.8 mmol) in ethanol (10 mL) and the mixture was stirred at 60 ℃ in a constant temperature incubator shaker for a specified period of time. After the completion of the reaction, water (20 mL) was added to the mixture to quench the reaction, the enzymes and the excess aldehydes were dissolved in an aqueous ethanol solution, and the desired products precipitated out. The products were collected by filtration without further purification.
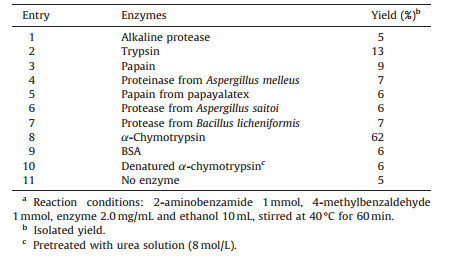
3. Results and discussionBased on our previous research, initial efforts were undertaken using 2-aminobenzamide and 4-methylbenzaldehyde as a model reaction, and ethanol was chosen as the reaction medium. In order to select the most efficient catalyst for the cyclocondensation, eight proteases were screened using the model system. As shown in Table 1, the best yield of 62% was achieved using α-chymotrypsin (Table 1, entry 8) while trypsin from bovin pancreas showed low activity in this reaction and only provided 13% yield (Table 1, entry 2). However, the other tested enzymes demonstrated no catalytic ability. Non-enzyme protein BSA (Bovine serum albumin) (Table 1, entry 9) and denatured α-chymotrypsin (Table 1, entry 10) were also used as controls to demonstrate the specific catalytic effect of the α-chymotrypsin, and both barely showed catalytic activity, producing the similar results as the blank control reaction (Table 1, entry 11). All the results clearly indicated that the catalytic ability of the α-chymotrypsin is responsible for the model reaction.
|
|
Table 1 The catalytic activities of different enzymes.a |
To improve the catalytic ability of the enzyme, we carried out some experiments focusing on the effect of reaction temperature, which plays a significant role in maintaining the stability and catalytic activity of enzymes. The range of temperature from 30 ℃ to 70 ℃ was screened for the α-chymotrypsin-catalyzed cyclocondensation reactions and the results were shown in Table 2, entries 1-4. The best yield of 89% was achieved at 60 ℃ (Table 2, entry 3) and higher temperature (70 ℃) gave lower yield probably due to the partial denaturation of the enzyme at higher temperatures. So, we chose 60 ℃ as an optimal temperature for the reaction. Next, the effect of enzyme loading on the achymotrypsin-catalyzed model reaction was investigated in order to further optimize the reaction conditions. As shown in Table 2, entries 5-9, the model reaction only gave the product in a low yield of 5% in the absence of enzyme. However, an obvious increase in the yield (77%) was detected when 0.5 mg/mL of α-chymotrypsin was added. After that, only a slight rising trend was observed with the larger catalyst dosage from 0.5 mg/mL to 2.5 mg/mL, thus, 2.0 mg/mL was chosen as the best enzyme loading.
|
|
Table 2 The effect of temperature and enzyme loading on the model reaction.a |
Some further experiments were performed to investigate the effects of the molar ratio of substrates on the α-chymotrypsin catalyzed model reaction. As shown in Table 3, increasing the molar ratio of 2-aminobenzamide to 4-methylbenzaldehyde from 1.0:1.2 to 1.0:1.8 led to a growing yield (from 90% to 98%) of the desired product and further increasing the molar ratio from 1.0:1.8 to 1.0:2.0 hardly improved the results. Thus, we chose the molar ratio of 2-aminobenzamide to 4-methylbenzaldehyde of 1.0:1.8 as the optimal proportion of the substrate for the reaction.
|
|
Table 3 The effect of molar ratio on the model reaction.a |
Finally, we further investigated the substrate scope and the limitation of the α-chymotrypsin-catalyzed cyclocondensation reaction by employing a wide range of aldehydes under the optimized conditions. The results are summarized in Table 4. It can be seen that the corresponding dihydroquinazolin-4(1H)-ones were obtained with good to excellent yields in all cases. Furthermore, various types of aromatic aldehydes with electrondonating groups reacted very well with 2-aminobenzamide under the optimized reaction conditions, as well as aromatic aldehydes containing electron-withdrawing substituents, such as the methyl-and bromo-substituted benzaldehydes afforded the corresponding products b and f in 98% and 96% yields, respectively. Generally, the reaction could proceed smoothly and was not influenced by substituents in the aromatic ring. To our delight, aliphatic aldehydes such as octyl aldehyde were compatible with the reaction conditions to give the desired product (n) in high yield of 98%. And hexaldehyde also afforded the corresponding product (m) in excellent yield although another 30 min of reaction time is required.
|
|
Table 4 Investigation of the reactant scope of the cyclocondensation reaction for synthesis of 2, 3-dihydroquinazolin-4(1H)-one derivatives.a |
4. Conclusion
In summary, we have succeeded in obtaining dihydroquinazolin-4(1H)-ones using α-chymotrypsin as a new biocatalyst. A wide range of aromatic aldehydes and aliphatic aldehydes could be used to give the products in excellent yields. Notably, The unnatural ability of α-chymotrypsin to catalyze a cyclocondensation reaction to prepare 2, 3-dihydroquinazolin-4(1H)-one derivatives was discovered for the first time. Compared with current chemical approaches, this enzymatic method is more environmental-friendly and sustainable using a biocatalyst from bio-degradable and inexpensive sources. Moreover, the catalyst α-chymotrypsin accommodated broad substrates and produced the desired products in high yields in a short period of time.
AcknowledgmentsWe gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 21262002, 21462001 and 21465002), the Program for Changjiang Scholars and Innovative Research Team in University (IRT13054), the Natural Science Foundation of Jiangxi No. 20142BAB203008), the Science and Technology Foundation of the Jiangxi Education Department (Nos. KJLD12006, GJJ14466 and KJLD14050).
| [1] | X.S. Wang, J. Zhou, S.J. Tu, Facile method for the combinatorial synthesis of 2, 2-disubstituted quinazolin-4(1H)-one derivatives catalyzed by iodine in ionic liquids. J. Comb. Chem. 12 (2010) 417–421. DOI:10.1021/cc900174p |
| [2] | M. Ghashang, S.S. Mansoor, K. Aswin, Synthesis of 2, 3-dihydroquinazolin-4(1H)-ones catalyzed by succinimide-N-sulfonic acid as a mild and efficient catalyst. Res. Chem. Intermed. 1 (2013) 1–14. |
| [3] | B.L. Xua, J.P. Chen, R.Z. Qiao, D.C. Fu, Facile and efficient synthesis of 2-substituted-N1-carbethoxy-2, 3-dihydro-4(1H)-quinazoliones in fluorous solvent. Chin. Chem. Lett. 19 (2008) 537–540. DOI:10.1016/j.cclet.2008.03.022 |
| [4] | V.B. Labade, P.V. Shinde, M.S. Shingare, A facile and rapid access towards the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones. Tetrahedron Lett. 54 (2013) 5578–5580. |
| [5] | M. Abdollahi-Alibeik, E. Shabani, Synthesis of 2, 3-dihydroquinazolin-4(1H)-ones catalyzed by zirconium (IV) chloride as a mild and efficient catalyst. Chin. Chem. Lett. 22 (2011) 1163–1166. |
| [6] | A. Shaabani, A. Maleki, H. Mofakham, Click reaction:highly efficient synthesis of 2, 3-dihydroquinazolin-4(1H)-ones. Synth. Commun. 38 (2008) 3751–3759. DOI:10.1080/00397910802213802 |
| [7] | M. Dabiri, P. Salehi, S. Otokesh, Efficient synthesis of mono-and disubstituted 2, 3-dihydroquinazolin-4(1H)-ones using KAl (SO4)2·12H2O as a reusable catalyst in water and ethanol. Tetrahedron Lett. 46 (2005) 6123–6126. DOI:10.1016/j.tetlet.2005.06.157 |
| [8] | M. Dabiri, P. Salehi, M. Baghbanzadeh, Silica sulfuric acid:an efficient reusable heterogeneous catalyst for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones in water and under solvent-free conditions. Catal. Commun. 9 (2008) 785–788. DOI:10.1016/j.catcom.2007.08.019 |
| [9] | R. Amin, T. Ashkan, Sulfamic acid as a reusable and green catalyst for efficient and simple synthesis of 2-substituted-2, 3-dihydroquinazolin-4(1H)-ones in water or methanol. Chin. Chem. Lett. 22 (2011) 1317–1320. DOI:10.1016/j.cclet.2011.06.008 |
| [10] | M. Abdollahi-Alibeik, E. Shabani, Nanocrystalline sulfated zirconia as an efficient solid acid catalyst for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones. J. Iranian Chem. Soc. 11 (2014) 351–359. DOI:10.1007/s13738-013-0306-5 |
| [11] | A. Rostami, B. Tahmasbi, H. Gholami, H. Taymorian, Supported N-propylsulfamic acid on magnetic nanoparticles used as recoverable and recyclable catalyst for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones in water. Chin. Chem. Lett. 24 (2013) 211–214. DOI:10.1016/j.cclet.2013.01.032 |
| [12] | J. Safari, S. Gandomi-Ravandi, Efficient synthesis of 2-aryl-2, 3-dihydroquinazolin-4(1H)-ones in the presence of nanocomposites under microwave irradiation. J. Mol. Catal. A:Chem. 390 (2014) 1–6. DOI:10.1016/j.molcata.2014.02.013 |
| [13] | J. Wu, X. Du, J. Ma, Preparation of 2, 3-dihydroquinazolin-4(1H)-one derivatives in aqueous media with β-cyclodextrin-SO3H as a recyclable catalyst. Green Chem. 16 (2014) 3210–3217. DOI:10.1039/c3gc42400f |
| [14] | Y. Parthasaradhi, C. Rakhi, S. Suresh, S.J. Tangenda, Polyethylene glycol (PEG-400) as a medium for novel andefficient synthesis of 2-phenyl-2, 3-dihydroquinazolin-4(1H)-onederivatives. Eur. J. Chem. 4 (2013) 462–466. DOI:10.5155/eurjchem.4.4.462-466.847 |
| [15] | H.R. Shaterian, M. Aghakhanizadeh, Brønsted acidic ionic liquids catalyze the preparation of 2, 3-dihydroquinazolin-4(1H)-one derivatives. Res. Chem. Intermed. 40 (2014) 1655–1668. DOI:10.1007/s11164-013-1071-x |
| [16] | H.H. Li, Y.H. He, Y. Yuan, Z. Guan, Nuclease p1:a new biocatalyst for direct asymmetric aldol reaction under solvent-free conditions. Green Chem. 13 (2011) 185–189. DOI:10.1039/C0GC00486C |
| [17] | J.F. Cai, Z. Guan, Y.H. He, The lipase-catalyzed asymmetric C-C Michael addition. J. Mol. Catal. B:Enzym. 68 (2011) 240–244. DOI:10.1016/j.molcatb.2010.11.011 |
| [18] | T. Purkarthofer, K. Gruber, M. Gruber-Khadjawi, A biocatalytic Henry reaction-the hydroxynitrile lyase from Hevea brasiliensis also catalyzes nitroaldol reactions. Angew. Chem. Int. Ed. 45 (2006) 3454–3456. DOI:10.1002/(ISSN)1521-3773 |
| [19] | H.R. Safaei, M. Shekouhy, V. Shafiee, M. Davoodi, Glycerol based ionic liquid with a boron core:a new highly efficient and reusable promoting medium for the synthesis of quinazolinones. J. Mol. Liq. 180 (2013) 139–144. DOI:10.1016/j.molliq.2013.01.013 |
| [20] | A.V. Dhanunjaya Rao, B.P. Vykunteswararao, T. Bhaskarkumar, Sulfonic acid functionalized Wang resin (Wang-OSO3H) as polymeric acidic catalyst for the eco-friendly synthesis of 2, 3-dihydroquinazolin-4(1H)-ones. Tetrahedron Lett. 56 (2015) 4714–4717. DOI:10.1016/j.tetlet.2015.06.004 |
 2017, Vol. 28
2017, Vol. 28