b Department of Chemistry, M. V. Lomonosov Moscow State University, Moscow 119991, Russian Federation
Phthalocyanines have attracted a lot of interest in research because of their unique linear and non-linear optical and photophysical properties [1-5]. Aggregation of macrocycles is a well-known phenomenon, giving rise to a decrease of light absorption, fluorescence quantum yields and reduction of the photosensitising efficiency [6]. Earlier we have clearly demonstrated that aggregation can be controlled by purposeful formation of stable J-type dimeric structures with a given location of the macrocycles [7, 8]. Unlike monomeric derivatives, chemically and thermally stable J-type phthalocyanine dimers, instead of typical uncontrolled aggregation, cause a formation of ordered ensembles of nanoscale level (nanoaggregation), that may indicate preservation of the optical properties in monolayers or thin films. The possibility of forming nanoensembles on the basis of phthalocyanine J-type dimers depends on the size of the peripheral substituents, the presence of functional groups [8] as well as the geometry of dimeric molecules which is determined by the nature of the complexed metal or the lack of it, as shown in this study.
2. ExperimentalPreparation of bis (2-hydroxy-9(10), 16(17), 23(24)-tri-tert-butylphthalocyanine) (2): Dimeric complex 1 [9] (120 mg, 0.08 μmol) was completely dissolved in concentrated sulphuric acid (25 mL), then the resulting dark brown solution was poured onto ice to precipitate target metal-free dimer 2 (93 mg, yield-80%). MALDI-TOF (matrix-2, 5-dihydroxybenzoic acid): 1397.6946 [M + H]+, calcd. for [C88H84N16O2] 1396.6963. 1H NMR (CCl4 + 5% DMSO-d6): δ -1.49--0.82 (m, 4H, NH), 1.22-2.49 (m, 54H, C (CH3)3), 7.22-10.00 (m, 24H, Ar). UV/vis (THF), λmax(log ε, nm): 291 (4.37), 339 (4.64), 610 (4.24), 669 (4.73), 703 (4.67), 715 (4.68). FT-IR (CCl4): 4000-3087 (NH, OH), 2975-2846 (CAr-H), 1714, 1590 (NH), 1460, 1376 (CAr-H), 1004 (OH), 752 (CAr-H). Anal. Calcd. for C88H84N16O2: C, 75.62; H, 6.06; N, 16.03%. Found: C, 75.60; H, 6.11; N, 16.08%. Fluorescence data: excitation λexc (THF)=600 nm, emission λem=705 nm, Stokes' shift 5 nm, ФF=0.008.
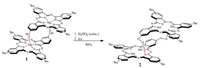
3. Results and discussionWe first demonstrate the method used to obtain the metal-free slipped-cofacial phthalocyanine dimers (Scheme 1).
|
Download:
|
| Scheme 1. Preparation of metal-free slipped-cofacial J-type phthalocyanine dimer. | |
It has been found that removing magnesium is nearly quantitative in concentrated sulphuric acid. In the MALDI-TOF mass spectrum of self-assembled compound 2, only one molecular ion peak, with m/z 1397.6946 ([M + H]+), was found. In the 1H NMR spectrum the aromatic proton signals broaden and were observed in the range of 7.22-10.00 ppm, demonstrating significant intermolecular interactions of the macrocycles. DFT optimisation of the dimeric structure 1 after removing of magnesium has led to slight moving of the macrocycles to form assembly 2, in which hydrogen bonds appear between the H atoms of the OH-groups of the first macrocycle and the isoindoline nitrogen atoms of another one (Fig. 1b). According to natural bond orbital (NBO) analysis, the stabilisation energy between the lone pairs of N atoms (donor) and OH bonds (acceptor), i.e. nN→σOH*, corresponds to 6-11 kcal/mol.
The dimeric phthalocyanine 2 was characterised by UV/vis and fluorescence emission spectra in comparison with the starting magnesium complex 1 (Fig. 1a).

|
Download:
|
| Figure 1. UV/vis (a) and fluorescence emission (λexc=600 nm) spectra (in THF solution), DFT optimised structures (b) of the models based on the dimeric compounds 1 and 2 (tert-butyl groups were omitted for clarity), FE-SEM images of dimeric complex 1 (c) and metal-free dimer 2 (d). | |
In the case of dimeric complex 1, an additional band at 695 nm was observed, which is red-shifted towards the Q-band by 21 nm (in THF). This band is referred to as the J-band and describes the specific parallel shifting of the macrocycles in dimers and aggregates of J-type to an angle of less than 54.7°, which is called the slip angle [10]. For the metal-free dimer 2 the appearance of an additional band at 715 nm is observed, which can be also assigned to the J-band. Thus, compound 2 can be regarded as a dimer of Jtype, similar to complex 1, from which it is derived. This is also indicated by the almost same fluorescence quantum yield of 2 (ФF=0.008), in respect to complex 1 (ФF=0.014). The emission band of 2 is red-shifted in comparison with starting dimer 1 (Fig. 1a, inset). Additional spectral experiments were provided to show that the J-dimers 1 and 2 cannot produce monomeric species in solutions.
Dimeric phthalocyanines 1 and 2 have high chemical stability. Addition of coordinating reagents does not lead to dissociation or destruction of their structures that was confirmed by special studies. According to differential scanning calorimetric analysis, dimers 1 and 2 are stable up to 600 degrees, wherein the metal-free dimer 2 is more resistant than the parent complex 1. In the solidstate, slipped-cofacial dimeric phthalocyanines 1 and 2 have a tendency to yield nanoensembles, as was evidenced by FE-SEM investigations (Fig. 1c, d). In the case of magnesium complex 1, the formation of rings with a diameter of 175 nm and a wall thickness of 20 nm was observed, that corresponds to the package of 100 molecules in layers of 8 molecules (Fig. 1c). At the macroscopic level, these rings combine together to give visually distinguishable zones with widths of about 600 nm and the same distance between them. Metal-free dimer 2 (Fig. 1d) also demonstrates second order nanoaggregation; nanoscale objects are presented as rings with a diameter of 225 nm (280 molecules) and a wall thickness of 22 nm (10 molecules). These nanoaggregates are elongated into thin threads with a distance between them of about 2 μm and a thickness of one unit.
The unusual association of the dimeric molecules can be explained in the terms of π-π interactions and formation of intermolecular hydrogen bonds between adjacent molecules. Two possible mechanisms were suggested previously to describe the features of nanoring genesis [8]. Despite the fact that the investigations are being continued, at the moment we can note the significant role of the nature of peripheral substituents. The study of aggregation of slipped-cofacial dimeric molecules in the solid-state is extremely important task for applications because it allows us to find ways to control this phenomenon, resulting in not only the preservation, but even the enhancement of important spectral and photophysical properties of materials based on phthalocyanines and their analogues. Metal-free slipped-cofacial J-type phthalocyanine dimers could be regarded as valuable precursors to yield a wide range of J-type dimeric complexes that cannot be obtained by the direct method [7, 8].
4. ConclusionsMetal-free slipped-cofacial phthalocyanine dimer 2 was derived from the dimeric magnesium phthalocyanine (complex 1) under action of concentrated sulphuric acid. According to the NBO calculations, the stabilisation of the dimeric structure is estimated to be within the range of 6-11 kcal/mol. In the UV/vis spectrum of the metal-free dimer, a splitting of the bathochromic component of the Q-band and the absence of the H-band (approximately at 650 nm) were detected, wherein both dimers 1 and 2 exhibit similar fluorescence properties. This indicates that the J-type dimeric complex produces the same type of metal-free derivative. Due to peripheral tert-butyl substituents in the dimeric structures, we were able to find second order nanoaggregation in the solid phase. Thermoanalytical studies have shown high stability of the dimers up to 600 ℃.
AcknowledgmentsThis research was supported by the council under the President of the Russian Federation for State Support of Young Scientists (No. MD-3738.2015.3), as well as by the Russian Foundation for Basic Research (Nos. 16-33-60031 and 15-33-21012). The authors also thank Dr. Alexander Dzuban and Dr. Pavel Tarakanov for implementation of thermoanalytical and fluorescence studies, as well as the Joint Supercomputer Centre of RAS (www.jscc.ru) for providing computing resources. Electron microscopy characterisation was performed in the Department of Structural Studies of the Zelinsky Institute of Organic Chemistry, Moscow.
| [1] | H.S. Nalwa, Supramolecular photosensitive and electroactive materials, Academic Press, San Diego, 2001. |
| [2] | E. Güzel, A. Atsay, S. Nalbantoglu, Synthesis, characterization and photodynamic activity of a new amphiphilic zinc phthalocyanine. Dyes Pigm. 97 (2013) 238–243. DOI:10.1016/j.dyepig.2012.12.027 |
| [3] | J.D. Zhang, F.L. Lu, H.C. Huang, Near infrared electrochromism of lutetium phthalocyanine. Synth. Met. 148 (2005) 123–126. DOI:10.1016/j.synthmet.2004.09.027 |
| [4] | E.A. Lukyanets, V.N. Nemykin, The key role of peripheral substituents in the chemistry of phthalocyanines and their analogs. J. Porphyr. Phthalocyanines 14 (2010) 1–40. DOI:10.1142/S1088424610001799 |
| [5] | V.N. Nemykin, E.A. Lukyanets, The key role of the peripheral substituents in the chemistry of phthalocyanines, in:K.M. Kadish, K.M. Smith, R. Guilard (Eds.), Handbook of porphyrin science, , World Scientific, Singapore, 2010, pp. 1-323. |
| [6] | M. Sevim, M.N. Yaraşir, A. Koca, M. Kandaz, Novel scorpion type phthalocyanine chemosensors for detection of selective-metal ion by inducing H-and J-aggregations in solution; synthesis, characterization and electrochemistry. Dyes Pigm. 111 (2014) 190–201. DOI:10.1016/j.dyepig.2014.05.036 |
| [7] | A. Yu, . Tolbin, V.E. Pushkarev, I.O. Balashova, A highly stable double-coordinated 2-hydroxy-tri (tert-butyl)-substituted zinc phthalocyanine dimer:synthesis, spectral study, thermal stability and electrochemical properties. New J. Chem. 38 (2014) 5825–5831. DOI:10.1039/C4NJ00692E |
| [8] | Yu.A. Tolbin, A.V. Dzuban, V.I. Shestov, Peripheral functionalisation of a stable phthalocyanine J-type dimer to control the aggregation behaviour and NLO properties:UV-Vis, fluorescence, DFT, TDHF and thermal study. RSC Adv. 5 (2015) 8239–8247. DOI:10.1039/C4RA15239E |
| [9] | Yu.A. Tolbin, V.B. Sheinin, O.I. Koifman, L.G. Tomilova, Synthesis of stable dimeric phthalocyanine J-type complexes and investigation of their nucleophilic properties. Macroheterocycles 8 (2015) 150–155. DOI:10.6060/mhc150454t |
| [10] | T. Nyokong, E. Antunes, Photochemical and photophysical properties of metallophthalocyanines, in:K.M. Kadish, K.M. Smith, R. Guilard (Eds.), Handbook of porphyrin science, , World Scientific Press, Singapore, 2010, pp. 247-357. |
 2017, Vol. 28
2017, Vol. 28 


