Dimethyl carbonate (DMC) is a green chemical, which has been widely utilized in chemical synthesis and solvents [1, 2]. Due to the toxicity and hazards of phosgene, non-phosgene processes of manufacturing DMC offer green alternatives in recent years [3, 4]. Among them, vapor-phase oxidative carbonylation of methanol which utilizes methanol, O2 and CO as reactants to synthesize DMC has a promising prospect, according to the principles of green chemistry [2].
CuCl2 loaded on various supports, such as silica and activated carbon (AC) has exhibited an excellent initial catalytic performance in oxidative carbonylation of methanol [5-7]. Among the supports, AC is highly attractive because of its high surface area, low price and conveniently modified surface [8]. However, the loss of chlorine is unavoidable, which would cause deactivation and corrosion [7]. King prepared Cu (I) Y catalyst with good activity and little deactivation via solid-state ion-exchange method [9], which demonstrated that the presence of chlorine was not essential in this reaction system. Since then, chloride-free catalysts have attracted much attention. In a previous study of Richter and coworkers, the existence of CuOx in CuY catalyst was proved to be favorable to oxidization of methanol, resulting in acceleration of DMC [10]. Huang et al. prepared CuY catalysts by ammonia evaporation method and the yield of DMC had a correlation with the proportion of cuprous species, including both Cu+ ions and Cu2O [11].
Inspired by the chloride-free catalysts supported on zeolite, CuOx/AC prepared from chloride-free Cu (Ⅱ) precursors has been exploited. By evidence from experiment results, Cu2O has been proposed as the main active site in some reported work [12, 13]. Through theoretical investigation on Cu2O (111), Wang and coworkers demonstrated that CO insertion to methoxide species was the main pathway contributing to DMC formation [14, 15]. To our knowledge, thermolysis of chloride-free Cu (Ⅱ) precursors supported on AC, such as Cu (CH3COO)2 and Cu2(NO3)(OH)3, was intensively studied to synthesize CuOx/AC catalyst [13, 16]. However, high temperature ( > 623 K) was necessary and the nature of Cu species was heavily dependent on the surface chemical properties of AC. Solvothermal reduction route, in which ethylene glycol (EG) was utilized as a reducing agent, was also employed to obtain Cu2O/AC in our previous work [17]. Although this process could be achieved at a lower temperature, the consumption of reducing agent in the process was excessive (100 mL EG/gcat). Besides, we found it difficult to reduce the particle size of Cu2O, which might result in the limited activity. Thus, it is desirable to develop a new method to fabricate Cu2O/AC catalysts with favorable catalytic performance.
In this study, we prepared CuOx/AC composite by vapor-phase methanol reduction under mild conditions (413 K, 0.5 MPa, same to reaction conditions and 36 mL methanol/gcat). The obtained CuOx/AC catalyst exhibited an enhanced catalytic performance in oxidative carbonylation reaction of methanol compared with the conventional methods. On the basis of characterization results from XRD, TEM and XPS, the excellent catalytic performance was also interpreted.
2. Experimental 2.1. Catalyst preparationCuOx/AC-T (Thermolysis method): As literature reported, pretreatment of AC with nitric acid solution led to the welldispersed CuOx particles [12]. Therefore, we pretreated AC with nitric acid solution under optimized concentration according to Yan et al. [17]. AC (40-70 mesh) was pretreated with nitric acid solution (11 mol/L) at 333 K for 4 h, then washed and dried overnight. 2 g modified AC was added to 100 mL of aqueous Cu (CH3COO)2 (0.034 mol/L) and the mixture was stirred vigorously for 4 h. Then the solvent was evaporated off and the residual solid was dried overnight. This catalyst precursor was denoted as Cu (Ⅱ)/ AC-PA. Ammonium hydroxide was added to 100 mL of aqueous Cu (NO3)2 (0.034 mol/L) dropwise until pH 8. Then the modified AC (2 g) was added to the liquid under stirring. After washing and drying, this catalyst precursor was denoted as Cu (Ⅱ)/AC-PN. Cu (Ⅱ)/ AC-PA and Cu (Ⅱ)/AC-PN was treated in a N2 flow at 473 K and 623 K for 4 h, respectively. The obtained samples were denoted as CuOx/AC-TA and CuOx/AC-TN.
CuOx/AC-V (Vapor-phase methanol reduction method): The conversion of Cu (Ⅱ)/AC-PA to CuOx/AC was conducted by in-situ vapor-phase methanol treatment. 0.2 g precursor Cu (Ⅱ)/AC-PA was placed in the reactor system which was heated to the desired temperature at a rate of 3 K/min in N2. Methanol was fed into the reactor by a syringe pump via an evaporator at a speed of 1.8 mL/h for 4 h. The obtained sample was denoted as CuOx/AC-V. After reduction, the reactor system was adjusted to reaction temperature and maintained for 10 min before reaction.
2.2. CharacterizationX-ray diffraction (XRD) measurements were performed using a Bruker/D8-Focus diffractometer in the range of 20° to 80° with a scanning rate of 8°/min. The products of vapor-phase methanol reduction were collected through condenser and subsequently monitored by a GC-MS apparatus (Agilent 7890B and 5977-MSD). Elemental analysis of copper was performed on an inductively coupled plasma optical emission spectroscope (ICP-OES) (VistaMPX, Varian). Transmission electron microscopy (TEM) images were recorded on a Jem-2100f apparatus. X-ray photoelectron spectroscopy was carried out on a Perkin-Elmer PHI 1600 ESCA system using monochromatic Al Kα (E=1486.6 eV) as the excitation source under UHV (6.67 × 10-7 Pa).
2.3. Catalytic measurementsCatalytic measurements were performed in a continuous microreactor system (WFS-3015) with a feed of 28.5 vol% vaporized methanol, 65.5 vol% CO and 6.0 vol% O2 (the total flow rate 66.5 mL/min). 0.2 g catalyst sample was positioned in the reactor furnace for each run. The reaction pressure was maintained at 0.5 MPa. Before reaction, the reactor was heated at a rate of 3 K/min to 413 K. The reaction products were analyzed by an online coupled gas chromatograph (Agilent 7890A) and then detected by flame ionization detector (FID). Methanol conversion and product selectivity were calculated as described previously [17]. The analysis of reaction products was completed at 30 min intervals and the catalytic performance was obtained based on the average value of 3~5 h.
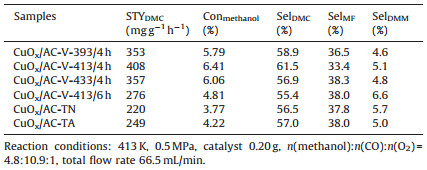
3. Results and discussionAs literature reported, Cu2O nanoparticles will be generated during the thermal treatment of copper salts supported on AC, owing to the decomposition of Cu precursors (e.g. Cu (CH3-COO)2·H2O). Therefore, in order to eliminate the impact of Cu (CH3COO)2·H2O thermolysis and confirm the reductive role of methanol, Cu (Ⅱ)/AC-PA treated with different calcination temperatures is monitored by XRD. As depicted in Fig. 1, no characteristic signals of Cu species are observed for the sample calcinated in N2 atmosphere at 413 K. It was also reported that only dehydration of Cu (CH3COO)2·H2O took place below 443 K in previous work [18]. Thus, Cu species are supposed to be highly dispersed in the form of copper acetate on the surface of AC. In other words, the transformation of Cu (CH3COO)2 to Cu2O does not occur at this temperature. When the calcination temperature is up to 473 K, peaks of Cu2O (JPCDS #05-0667) are emerged [19, 20]. After calcination at 523 K, the peaks assigned to Cu (JPCDS #04-0836) can be observed in the XRD pattern along with the characteristic peaks of Cu2O. When the thermolysis temperature is raised to 573 K, metallic Cu has already become the majority of Cu species with large particle size. Comparatively, characteristic peaks of Cu2O are appeared after vapor-phase methanol treatment at 413 K. Thus, by comparing the XRD pattern of the sample calcinated in N2 flow with that reduced by methanol at the same temperature, we could conclude that the generation of Cu2O is induced by the methanol reduction.

|
Download:
|
| Figure 1. XRD patterns of CuOx/AC composites prepared by thermolysis of Cu (CH3COO)2·H2O/AC at different temperatures and methanol treatment. | |
It has been demonstrated that Cu (Ⅱ) can be reduced by polyols in liquid phase, for instance, EG and diethylene glycol [21, 22]. However, few works have been reported about the reduction of Cu (Ⅱ) by methanol, especially vapor-phase methanol. To further investigate the reduction process, the products are collected through a condenser and subsequently monitored by a GC-MS apparatus. Signals of products with m/z of 18, 31, 45, 60 and 75 are detected, which can be ascribed to water, methanol, dimethyl ether, methyl formate and methyl acetate, respectively. Among these products, dimethyl ether is generated through dehydration of methanol. Methyl acetate is formed by reaction between methanol and acetate group. And methanol, serving as a reducing agent, is oxidized into methyl formate.
The obtained CuOx/AC composite is applied to catalytic synthetic process of DMC. In order to clarify the effects of preparation methods and different methanol reduction conditions, a comparative study of catalytic performance over different catalysts is carried out. The activity evaluation is conducted under the same reaction condition and the results are given in Table 1. It is apparent that the catalysts prepared through vapor-phase methanol reduction show the enhanced STY of DMC with the similar selectivity, in comparison with CuOx/AC-TA and CuOx/ACTN (249 mg g-1 h-1 and 220 mg g-1 h-1, respectively). The main by-product is methyl formate, whereas only a small amount of dimethoxymethane is generated. As for the catalysts prepared through vapor-phase methanol reduction, catalytic performance is obviously influenced by the reduction conditions: STY of DMC increases first and then decreases with increasing reduction temperature. Meanwhile, the extended reduction time results in the decline of DMC STY. The maximum DMC STY reaches up to 408 mg/(g·h) on CuOx/AC-V-413/4 h. In previous study of our group, Cu2O/AC catalyst could be prepared through EG reduction in liquid phase [17]. Compared with catalyst reduced by EG (STY 150 mg g-1 h-1), the catalytic performance over catalyst through the vapor-phase methanol reduction is also significantly enhanced.
|
|
Table 1 Catalytic performance of the CuOx/AC catalysts. |
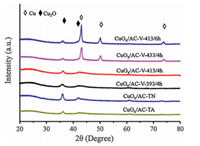
XRD is employed to investigate the influence of reduction methods and conditions on the crystalline phases of Cu species in CuOx/AC catalysts. As shown in Fig. 2, all the patterns exhibit the characteristic signal of Cu2O. Meanwhile, the characteristic peaks of Cu0 are emerged only in CuOx/AC-V-413/6 h and CuOx/AC-V-433/4 h, indicating that higher temperature and longer time promote the reduction of Cu2+ and Cu+ to Cu0. The crystallite sizes of Cu2O and Cu0 are calculated by Scherrer equation according to peaks at 36.4° and 50.4°, respectively. The corresponding results in Table 2 show that the particle size of Cu2O increases gradually from 6.4 to 11.4 nm with the increase of reduction temperature and time. Besides, the particle sizes of Cu0 in CuOx/AC-V-433/4 h and CuOx/AC-V-413/6 h are 11.3 and 13.4 nm, which are slightly bigger than corresponding Cu2O respectively, indicating the aggregation of Cu species during reduction. It is also worth noting that all the CuOx/AC-V samples possess the smaller particle size than CuOx/AC-TA and CuOx/AC-TN. Compared with catalyst reduced by EG (average crystallite size~25 nm) in our previous study, the dispersion of Cu2O nanoparticles is also significantly improved [17]. Therefore, it is inferred that vapor-phase methanol reduction is more favorable for higher copper dispersion in CuOx/ AC catalyst.
|
Download:
|
| Figure 2. XRD patterns for CuOx/AC catalysts prepared by different methods and methanol reduction conditions. | |
|
|
Table 2 Copper particle size and Cu valance distribution of the CuOx/AC catalysts. |
TEM provides a direct technique to characterize the dispersion of copper species. As shown in Fig. 3, it is easy to distinguish the distributed nanoparticles on the surface of AC in all samples. Although the average sizes of Cu particles characterized by TEM (listed in Table 2) are slightly larger than those obtained by XRD, the variation trend is consistent: compared to the thermolysis method, the vapor-phase methanol reduction benefits to the formation of smaller copper particles, especially at low temperature and short time. In addition, the increased reduction time and temperature result in the decline of Cu species dispersion. As literature reported, copper dispersion was proved to play a key role in the catalytic activity of oxidative carbonylation reaction [12]. Hence, the enhanced catalytic performance of CuOx/AC-V might be attributed to the high copper dispersion. In addition, in comparison with CuOx/AC-V-413/4 h, the larger Cu particle sizes of CuOx/AC-V-433/4 h and CuOx/AC-V-413/6 h also result in the decrease of DMC STY.

|
Download:
|
| Figure 3. TEM images of CuOx/AC catalysts (a, CuOx/AC-V-393/4 h; b, CuOx/AC-V-413/4 h; c, CuOx/AC-V-433/4 h; d, CuOx/AC-V-413/6 h; e, CuOx/AC-TN; f, CuOx/AC-TA). | |
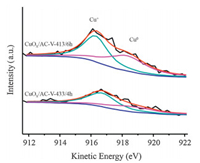
XPS characterization is applied to elucidate Cu valance distribution on the surface of CuOx/AC catalysts. Fig. 4a shows the spectra of CuOx/AC catalysts in the range of 925 to 965 eV. Except for CuOx/AC-V-413/6 h and CuOx/AC-V-433/4 h, the characteristic shakeup satellite peak of Cu2+ species at around 944.0 eV is observed, indicating the presence of Cu2+ in these samples [23, 24]. In general, Cu2p3/2 spectra can be fitted into two peaks around 932.9 and 934.5 eV, which are respectively assigned to (Cu+ + Cu0) and Cu2+ [23, 25, 26]. Based on the results of XRD that metallic Cu only emerges in the samples of CuOx/AC-V-413/6 h and CuOx/AC-V-433/4 h. Thus quantitative information about the valence state of Cu species for the CuOx/AC except CuOx/AC-V-413/6 h and CuOx/AC-V-433/4 h can be acquired from Gaussian fitting figures of Cu 2p3/2 spectra (shown in Fig. 4b). As it's difficult to distinguish Cu0 and Cu+ in Cu 2p3/2 spectra, we further calculate the ratios of Cu+ and Cu0 from the Cu LMM XAES spectra (shown in Fig. 5) to obtain quantitative analysis of Cu species in the samples of CuOx/AC-V-413/6 h and CuOx/AC-V-433/4 h. According to the literature, the Cu LMM XAES spectra are deconvolved into two peaks centered at 916.5 eV and 918.5 eV, which are attributed to Cu+ and Cu0 species, respectively [27-29]. The Cu valance distribution for all the samples are listed in Table 2. Since the total Cu contents of these samples are at the same level (CuOx/AC-V 10.1%, CuOx/AC-TA 10.3%, CuOx/AC-TN 10.6%), we can come to the conclusion that with the increased methanol treatment temperature and time, the Cu+ content first increases and then decreases. And among these samples, CuOx/AC-V-413/4 h possesses the highest content of Cu+ (70.9%).

|
Download:
|
| Figure 4. (a) Cu2p and (b) Cu2p3/2 XPS spectra of the CuOx/AC catalysts. | |
|
Download:
|
| Figure 5. Cu LMM XAES spectra of CuOx/AC-V-413/6 h and CuOx/AC-V-433/4 h. | |
As for Cu-zeolite catalyst, whether prepared by solid-state ion exchange or incipient wetness impregnation method, Cu+ ions located at exchange sites are recognized as active sites for methanol oxidative carbonylation reaction [30, 31]. Huang et.al also demonstrated that yield of DMC had a correlation with the proportion of Cu+ species [11]. As for the AC supported copper catalysts, Cu2O has been proved to be the main active site by both experimental results and theoretical calculation [13, 14, 17]. Through the investigation of calcination temperature, Li and coworkers also demonstrated that Cu2O possessed the best catalytic performance among all the Cu valance states [13]. Therefore, we suppose that the highest Cu+ content of CuOx/AC-V-413/4 h could also be reasonable for the enhanced catalytic performance. Although the Cu2O particle size of CuOx/AC-V-393/4 h is smaller than that of CuOx/AC-V-413/4 h, DMC STY raises as the increase of Cu+ content.
4. ConclusionIn summary, CuOx/AC composite is successfully prepared by vapor-phase methanol reduction under mild condition. And the prepared catalysts display an enhanced catalytic activity for DMC formation in comparison with conventional methods, including thermolysis and solvothermal reduction. Based on the characterization results, the enhanced catalytic activity is mainly attributed to better copper dispersion and higher Cu+ content. Moreover, methanol treatment conditions have significant influences on the catalytic activity. As the vapor-phase methanol reduction process is energy-efficient and solvent-free, the present approach is also expected to introduce other metal oxides into various porous supports.
AcknowledgmentFinancial support from the National Natural Science Foundation of China (Nos. 21325626, 21406120, U1510203) and the Postdoctoral Science Foundation of China (Nos. 2014M560181, 2015T80214) is gratefully acknowledged.
| [1] | M.S. Han, B.G. Lee, I. Suh, Synthesis of dimethyl carbonate by vapor phase oxidative carbonylation of methanol over Cu-based catalysts. J. Mol. Catal. AChem. 170 (2001) 225–234. DOI:10.1016/S1381-1169(01)00073-5 |
| [2] | S.Y. Huang, B. Yan, S.P. Wang, X.B. Ma, Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev. 44 (2015) 3079–3116. DOI:10.1039/C4CS00374H |
| [3] | J. Bian, X.W. Wei, L. Wang, Z.P. Guan, Graphene nanosheet as support of catalytically active metal particles in DMC synthesis. Chin. Chem. Lett. 22 (2011) 57–60. DOI:10.1016/j.cclet.2010.07.028 |
| [4] | S.P. Wang, W. Li, Y.Y. Dong, Y.J. Zhao, X.B. Ma, Effects of potassium promoter on the performance of PdCl2-CuCl2/AC catalysts for the synthesis of dimethyl carbonate from CO and methyl nitrite. Chin. Chem. Lett. 26 (2015) 1359–1363. DOI:10.1016/j.cclet.2015.06.008 |
| [5] | D.N. Briggs, G. Bong, E. Leong, Effects of support composition and pretreatment on the activity and selectivity of carbon-supported PdCunClx catalysts for the synthesis of diethyl carbonate. J. Catal. 276 (2010) 215–228. DOI:10.1016/j.jcat.2010.08.004 |
| [6] | B. Yan, S.Y. Huang, Q.S. Meng, Ordered mesoporous carbons supported Wacker-type catalyst for catalytic oxidative carbonylation. AIChE J. 59 (2013) 3797–3805. DOI:10.1002/aic.14091 |
| [7] | P.B. Zhang, Z. Zhang, S.P. Wang, X.B. Ma, A new type of catalyst PdCl2/Cu-HMS for synthesis of diethyl carbonate by oxidative carbonylation of ethanol. Catal. Commun. 8 (2007) 21–26. DOI:10.1016/j.catcom.2006.05.018 |
| [8] | F. Rodríguez-reinoso, The role of carbon materials in heterogeneous catalysis. Carbon 36 (1998) 159–175. DOI:10.1016/S0008-6223(97)00173-5 |
| [9] | S.T. King, Reaction mechanism of oxidative carbonylation of methanol to dimethyl carbonate in Cu-Y Zeolite. J. Catal. 161 (1996) 530–538. DOI:10.1006/jcat.1996.0215 |
| [10] | M. Richter, M.J.G. Fait, R. Eckelt, Oxidative gas phase carbonylation of methanol to dimethyl carbonate over chloride-free Cu-impregnated zeolite Y catalysts at elevated pressure. Appl. Catal. B 73 (2007) 269–281. DOI:10.1016/j.apcatb.2006.11.015 |
| [11] | S.Y. Huang, J.J. Zhang, Y. Wang, Insight into the tunable CuY catalyst for diethyl carbonate by oxycarbonylation:preparation methods and precursors. Ind. Eng. Chem. Res. 53 (2014) 5838–5845. DOI:10.1021/ie500288g |
| [12] | G.Q. Zhang, Z. Li, H.Y. Zheng, Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol. Appl. Catal. B 179 (2015) 95–105. DOI:10.1016/j.apcatb.2015.05.001 |
| [13] | Z. Li, C.M. Wen, R.Y. Wang, H.Y. Zheng, K.C. Xie, Chloride-free Cu2O/AC catalyst prepared by pyrolysis of copper acetate and catalytic oxycarbonylation. Chem. J. Chin. Univ. 30 (2009) 2024–2031. |
| [14] | R.G. Zhang, H.Y. Liu, L.X. Ling, Z. Li, B.J. Wang, A DFT study on the formation of CH3O on Cu2O (111) surface by CH3OH decomposition in the absence or presence of oxygen. Appl. Surf. Sci. 257 (2011) 4232–4238. DOI:10.1016/j.apsusc.2010.12.026 |
| [15] | R.G. Zhang, L.Z. Song, B.J. Wang, Z. Li, A density functional theory investigation on the mechanism and kinetics of dimethyl carbonate formation on Cu2O catalyst. J. Comput. Chem. 33 (2012) 1101–1110. DOI:10.1002/jcc.v33.11 |
| [16] | R.Y. Wang, Z. Li, H.Y. Zheng, K.C. Xie, Preparation of chlorine-free Cu/AC catalyst and its catalytic properties for vapor phase oxidative carbonylation of methanol. Chin. J. Catal. 31 (2010) 851–856. |
| [17] | B. Yan, S.Y. Huang, S.P. Wang, X.B. Ma, Catalytic oxidative carbonylation over Cu2O nanoclusters supported on carbon materials:the role of the carbon support. Chem. Cat. Chem. 6 (2014) 2671–2679. |
| [18] | K.L. Zhang, J.H. Hong, G.H. Cao, The kinetics of thermal dehydration of copper (Ⅱ) acetate monohydrate in air. Thermochim. Acta. 437 (2005) 145–149. DOI:10.1016/j.tca.2005.06.038 |
| [19] | H.Z. Bao, W.H. Zhang, Q. Hua, Crystal-plane-controlled surface restructuring and catalytic performance of oxide nanocrystals. Angew. Chem. Int. Ed. 50 (2011) 12294–12298. DOI:10.1002/anie.v50.51 |
| [20] | Q. Hua, T. Cao, X.K. Gu, Crystal-plane-controlled selectivity of Cu2O catalysts in propylene oxidation with molecular oxygen. Angew. Chem. Int. Ed. 53 (2014) 4856–4861. DOI:10.1002/anie.201402374 |
| [21] | C. Feldmann, H.O. Jungk, Polyol-mediated preparation of nanoscale oxide particles. Angew. Chem. Int. Ed. 40 (2001) 359–362. DOI:10.1002/(ISSN)1521-3773 |
| [22] | X.Y. Yan, X.L. Tong, Y.F. Zhang, Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chem. Commun. 48 (2012) 1892–1894. DOI:10.1039/c2cc17537a |
| [23] | J.H. Kou, C.H. Lu, W.H. Sun, L. Zhang, Z.Z. Xu, Facile fabrication of cuprous oxidebased adsorbents for deep desulfurization. ACS Sustain. Chem. Eng. 3 (2015) 3053–3061. DOI:10.1021/acssuschemeng.5b01051 |
| [24] | J.L. Gong, H.R. Yue, Y.J. Zhao, Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 134 (2012) 13922–13925. DOI:10.1021/ja3034153 |
| [25] | L. Wang, Y.F. Feng, Y.H. Zhang, Effect of original activated carbon support and the presence of NOx on CO oxidation over supported Wacker-type catalysts. Fuel 96 (2012) 440–445. DOI:10.1016/j.fuel.2011.12.005 |
| [26] | S.P. Wang, W. Li, Y.Y. Dong, Y.J. Zhao, X.B. Ma, Dimethyl carbonate synthesis from methyl nitrite and CO over activated carbon supported Wacker-type catalysts:the surface chemistry of activated carbon. Catal. Commun. 72 (2015) 43–48. DOI:10.1016/j.catcom.2015.09.005 |
| [27] | Q. Hua, T. Cao, H.Z. Bao, Z.Q. Jiang, W.X. Huang, Crystal-plane-controlled surface chemistry and catalytic performance of surfactant-free Cu2O nanocrystals. Chem. Sus. Chem. 6 (2013) 1966–1972. DOI:10.1002/cssc.v6.10 |
| [28] | Z. Chen, D. Mochizuki, M.M. Maitani, Y. Wada, Facile synthesis of bimetallic Cu-Ag nanoparticles under microwave irradiation and their oxidation resistance. Nanotechnology 24 (2013) 265602. DOI:10.1088/0957-4484/24/26/265602 |
| [29] | T. Ghodselahi, M.A. Vesaghi, A. Shafiekhani, A. Baghizadeh, M. Lameii, XPSstudy of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 255 (2008) 2730–2734. DOI:10.1016/j.apsusc.2008.08.110 |
| [30] | Y.H. Zhang, D.N. Briggs, E. de Smit, A.T. Bell, Effects of zeolite structure and composition on the synthesis of dimethyl carbonate by oxidative carbonylation of methanol on Cu-exchanged Y, ZSM-5, and Mordenite. J. Catal. 251 (2007) 443–452. DOI:10.1016/j.jcat.2007.07.018 |
| [31] | Y.H. Zhang, A.T. Bell, The mechanism of dimethyl carbonate synthesis on Cuexchanged zeolite Y. J. Catal. 255 (2008) 153–161. DOI:10.1016/j.jcat.2008.01.033 |
 2017, Vol. 28
2017, Vol. 28