CeO2 is known for its widely application in heterogeneous catalysis, either as the catalyst or the support [1-3], because of its low redox potential [4], versatile acid and base properties [5] and high oxygen storage capability [6]. Three low-index crystal planes ({1 1 1}, {1 0 0} and {1 1 0}) exposed on ceria are affected by its morphology [7-13].
The direct synthesis of DMC from CO2 and methanol over the ceria catalyst appears to be a promising pathway because of its implications in CO2 fixation and sustainable development [12, 14, 15]. Some researchers [16, 17] have proposed a mechanism of mono-dentate methyl carbonate (MMC) as an intermediate product. Michele Aresta [18] thought the CO2 reacts directly with the methoxy group on the catalyst, while B.A.V. Santos [19] believed both CO2 and CH3OH are adsorbed on the ceria surface before they react with each other. It is worth noting that the latter viewpoint was supported by kinetic studies but no other characterizations for the reaction or catalyst were reported.
In this paper, three well-shaped cerium oxides exposing different surfaces were prepared to investigate the mechanism of DMC synthesis using CO2 and methanol. In situ FTIR was used to analyze the nature of the active sites needed for every step and explain the different catalytic performance on different crystal planes.
2. ExperimentalCatalyst preparation: Cerium oxides were prepared by a hydrothermal method. 480 mmol NaOH and 4 mmol Ce (NO3)3·6H2O were dissolved in 70 and 10 mL of deionized water. The mixed solution was stirred for 30 min to produce a purple slurry. Then the slurry was transferred into a Teflon-lined stainless autoclave and heated at 373 K and 453 K for 24 h. After the hydrothermal treatment, precipitates were collected by filtration, washed with deionized water and ethanol several times and dried at 353 K for 24 h. The samples were labeled as rod and cube, respectively. For CeO2 octahedron, 0.15 mmol Na3PO4 and 20 mmol Ce (NO3)3·6H2O were used, and the hydrothermal temperature was changed to 433 K. The sample was labeled as octa.
Catalyst characterization: The morphology of CeO2 samples was characterized through TEM and HRTEM operated at an accelerating voltage of 200 kV. FTIR measurements were performed on a Nicolet 6700 spectrometer (Thermo Fisher Scientific) equipped with an MCT detector. Four scans were averaged at a resolution of 4 cm-1. The sample was pressed into a disk and put into a homemade reaction cell with ZnSe windows. The cell temperature was controlled by a programmable temperature controller. Before the exposure to an adsorbent, the disk with catalyst was pretreated at 673 K in flowing He stream (15 mL/min) for 1 h and then cooled to 413 K (reaction temperature). CH3OH was brought into the cell with 15 mL/min He stream for 0.5 h through a saturator, which was kept at 278 K during the adsorption of methanol, followed by pure He stream for 0.5 h for the desorption of methanol. DMC was injected into the reaction cell using a syringe (0.2 mL). The flow rate of CO2 and He was set as 10 mL/min and 15 mL/min, respectively, controlled by a mass flow controller. He was deoxidized by a deoxidizing tube and passed through molecular sieves to remove water. The whole process was conducted at atmospheric pressure. And all infrared spectra exhibited were difference spectra referenced to the background spectrum collected at 413 K after pretreatment.
The synthesis of DMC from CH3OH and CO2: In a typical procedure of DMC synthesis, 15 mL CH3OH and 0.1 g catalyst were put into a stainless steel autoclave. Then the autoclave was filled with 5 MPa CO2. The reactor was heated and stirred at 413 K for 3 h. After the reaction, 1-propanol was added as a standard substance to quantify the mixture when the reactor was cooled to room temperature. Products were then analyzed by GC (Agilent 4890 D) analysis. DMC was the only product observed in our work.
3. Results and discussion 3.1. Structural and morphological of catalystsAs shown and discussed in our previous study [20], all the cerium oxide samples are of high purity, and the crystal planes exposed on different morphology ceria samples are determined. Only {1 1 1} crystal plane is observed on octa surface and only {1 0 0} on cube surface. Both {1 1 1} and {1 1 0} crystal planes are exposed on rod surface.
3.2. Catalytic activities of different ceria catalystsAlso shown in our previus work [20], the order of catalytic activity in DMC formation was summarized as: rod > octa > cube, and the rod showed much higher activity than other two samples. It is speculated that the different crystal planes play the most important role in this phenomenon.
3.3. In situ Fourier transform infrared spectroscopyFig. 1 shows the different IR spectra when methanol is used as a probe to different samples. For rod and cube, three negative peaks are shown at 3691, 3654 and 3590 cm-1 associated with terminal, bibridged, and tribridged -OH groups [21], respectively. Because -OH groups are on ceria surface originally, the bands associated with -OH stretching are included in the background that the FTIR spectra are based on. As being exposed to ceria, CH3OH reacts with surface -OH groups, which leads to the consumption of -OH groups on ceria samples. And as a result, negative peaks in -OH stretching region are observed. In the υ(CO) region, three bands grow at 1095, 1043 and 1010 cm-1, associated with on-top methoxy, bridging methoxy and three-coordinate methoxy species, respectively [22]. Upon adsorption, methanol reacts with the surface hydroxyl groups to form methoxy groups and release of water. This process is widely believed to be the first step in this reaction. Large negative peaks at 3500-3800 cm-1 and three peaks around 100-1100 cm-1 represent the -OH groups consumed and methoxy groups produced on cube and rod ceria surface. While for the octa, only a very small negative peak is observed at 3672 cm-1 with a band showing up at 1060 cm-1. It is speculated that there are not enough -OH groups on the octahedron ceria surface that can activate methanol to form methoxy groups. CO2 reacts with insufficient number of methoxy groups, leading to the low activity of octahedron for DMC formation.

|
Download:
|
| Figure 1. IR spectra following methanol desorption for 0.5 h on ceria catalysts at 413 K. | |
The distribution and assignment of CO2 adsorption species on three samples are shown in Fig. 2, based on both the IR experiment of CO2 (CO) adsorptions on ceria [23-26] and computational modeling studies [27, 28]. Generally, CO2 reacts with -OH groups on the surface and adsorbs as bicarbonates (1600, 1398, and 1215 cm-1), and CO2 can also react with surface lattice oxygen to mono-dentate (1353, 1465 cm-1), bridged (1242, and 1651 cm-1) and bidentate (1580, 1292 cm-1) carbonates. A small peak at 1693 cm-1 is associated with carboxylic acid. Because of the diverse atomic arrangement on the surface of different morphology ceria, the same type of carbonate species can also show slightly different vibrational frequencies when CO2 is conducted to different ceria nanoshapes [24]. Mono-dentate carbonate, bidentate carbonate and bicarbonate species can form on all the three samples. As number of -OH groups on ceria octahedron surface is much fewer than that on cube and rod, correspondingly, bicarbonates on ceria octahedron surface occupies less than that on cube and rod. It is noted that bidentate carbonates account for a large proportion on rod and octa, while only a small amount of bidentate carbonates can be observed on nanocube ceria. Because rod and octa ceria expose a large number of {1 1 0} and {1 1 1} planes, comparing with cubes exposing dominant {1 0 0} crystal planes. It is hypothesized that bidentate carbonates adsorb on ceria {1 1 0} and {1 1 1} crystal plane instead of {1 0 0}. On the other hand, bridged carbonates are merely observed on cube with {1 0 0} crystal plane, meaning that bridged carbonates adsorb only on ceria {1 0 0} crystal planes.
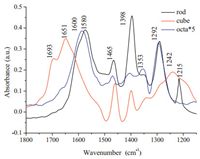
|
Download:
|
| Figure 2. IR spectra following CO2 adsorption for 0.5 h on ceria catalysts at 413 K. | |
Fig. 3a-3d demonstrates the evolution of CO2 adsorptions when CeO2 cube, rod and octa pre-adsorbed CO2 are exposed to CH3OH. For cube ceria in Fig. 3a, all large bands associated with different kinds of CO2 adsorptions (bicarbonate: 1200, 1399, 1600 cm-1, bridged carbonate: 1242, 1651 cm-1, mono-dentate carbonate: 1343, 1465 cm-1) disappear or reduce as CH3OH adsorbs on cube (line 1 to 7). It is noted that only a small band at 1346 cm-1 showed up, meaning little intermediate product is formed under this condition. Thus, it can be assumed that bicarbonate, mono-dentate carbonate and bridged carbonate species on CeO2 cube cannot react with -OCH3 groups to form any intermediate products.

|
Download:
|
| Figure 3. IR spectra taken during exposure of ceria pre-adsorbed CO2 to CH3OH at 413 K with (a) for cube, (b) for rod, (c) and (d) for octa. (As time goes by, the line in Fig. 3a and 3b changes from number 1 to number 7 and in Fig. 3c and Fig. 3d from 1 to 6. In Fig. 3a and 3b, the time scale from line 1 to line 7 is 0 s→2 min 17 s→2 min 25 s→3 min→6 min 54 s→9 min 8 s→30 min. The time scale from line 1 to 6 in Fig. 3c is 0 s→2 min 17 s→2 min 25 s→3 min→4 min 11 s→5 min 32 s and then in Fig. 3d from line 1 to 6 is 5 min 32 s→5 min 41 s→6 min 7 s→11 min 40 s→19 min 15 s→30 min. Line 6 in Fig. 3c and line 1 in Fig. 3d are the same one. Fig. 3d was obtained just after Fig. 3c in time scale). | |
For rod ceria in Fig. 3b, bands associated with -HCO3 (1600, 1398, and 1215 cm-1) disappear immediately under CH3OH condition and new bands are observed. At the same time, two bands associated with bidentate carbonate (1292 and 1576 cm-1) become higher. As the reaction proceeds, four bands (1292, 1346, 1461, and 1576 cm-1) grow together to the highest (line 1 to 4) and decrease together too (line 4 to 7). Bands at 1461 and 1346 cm-1 are associated with MMC [14, 21], and in some literatures [15, 18], the band at 1576 cm-1 also was assigned to MMC. It is possible that bands at 1576 and 1292 cm-1 all stand for MMC as they grow and reduce together with bands at 1461 and 1346 cm-1. The decrease of four bands (1292, 1346, 1461, and 1576 cm-1) from line 4 to 7 in Fig. 3b is because MMC is unstable under this condition and dissociates to form carbonate and methanol quickly. It is clear that some CO2 adsorpted on rod surface reacts with surface -OCH3 to form intermediate product MMC. Bands associated with bidentate carbonate show a slight excursion when growing, which can be explained by the interaction between bidentate carbonates and -OCH3 groups. From Fig. 3a, bicarbonates and mono-dentate carbonates are unable to react with -OCH3 groups to form MMC. It is hypothesized that bidentate carbonates are necessary for MMC formation. Also in Fig. 3a, only a small peak of bidentate carbonates (1292 cm-1) is observed on cube surface, which is necessary for MMC formation. As a result, bands associated with MMC on cube surface are barely observed.
Fig. 3 C D demonstrates the evolution of CO2 adsorptions when CeO2 octahedron pre-adsorbed CO2 is exposed to CH3OH. In Fig. 3c, all bands change similarly as bands in Fig. 3b. Two peaks associated with MMC (1461 and 1352 cm-1) are observed, and two original peaks associated with bidentate carbonate and MMC (1292 and 1581 cm-1) increase with the middle two bands at 1464 and 1340 cm-1 (Fig. 3c: line 1 to 6). After reaching the highest position, all these four peaks (1292, 1352, 1461, and 1581 cm-1) become smaller. However, they reduce slightly and then spectra become different compared to CO2 adsorbed on rod, and peaks associated with MMC is disturbed by other species. As observed in Fig. 3d, the peak at 1292 cm-1 continues to reduce, while the bands between 1350 cm-1 to 1400 cm-1 and 1550 cm-1 to 1600 cm-1 grow for the second time. Bands around 1354, 1371, 1561, and 1592 cm-1 can be assigned to a single and a two-fold coordinated formate species (m-OHCO and b-OHCO) [15, 21]. It can be explained that on the ceria octahedron surface, CO2 adsorbs as bidentate carbonates and mono-dentate carbonates, and only the former species can react with -OCH3 groups on octahedron surface to form intermediate product MMC. At the same time, formate species (Ce-OCHO) are formed in agreement with the reduction of ceria and four other bands (1354, 1371, 1561, and 1592 cm-1) grow again and cover the former ones. As no other bands are found on ceria rod and cube surface, it is possible that the formation of formates is more easily on octahedron surface, {1 1 1} crystal planes. Formate species (Ce-OCHO) are unstable under 413 K conditions, and the formates dissociate on the octahedron surface quickly, as a result bands associated with formates reduce with CH3OH steam pumping in.
Two bands at 1767 and 1781 cm-1 are characteristics of C=O groups in DMC. As no bands around 1770 cm-1 can be observed in Fig. 3 during CO2 and CH3OH adsorption, it is obvious that the reaction between MMC with another CH3OH to form DMC is the rate-determining step in the synthesis. Ceria nanorods, which show the highest catalytic activity, are exposed to the final product, DMC, to study the reverse reaction. In Fig. 4, bands at 1292, 1767 and 1781 cm-1 are associated with physically absorbed DMC, and all of these bands reduce immediately after DMC is injected in, which means DMC dissociate immediately on ceria nanorods. However, the peak at 1292 cm-1 still exists when peaks around 1767 and 1781 cm-1 already disappeared, at the same time other bands associated with MMC (1346, 1461, and 1570 cm-1) show up and increase (line 2-7). It appears that the band at 1292 cm-1 is associated with DMC and DMC dissociative adsorption, MMC. Bands related to bicarbonate (1215, 1400, and 1600 cm-1) are observed immediately and bands of formate (1354, 1371, and 1560 cm-1) show up quite late (line 7).
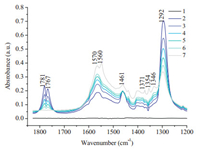
|
Download:
|
| Figure 4. IR spectra taken during exposure of rod to DMC at 413 K. (As time goes by the line changes from number 1 to number 7, the number 2 line is obtained just after DMC was injected into the reaction cell.). | |
Therefore, the small number of -OH groups on octahedron surface can only activate limited CH3OH to form -OCH3 groups on its {1 1 1} crystal planes and release H2O, so CO2 adsorptions can only react with these insufficient number of methoxy groups to form intermediate product MMC. As a result, the catalytic activity of octahedron is low. Ceria cubes have a large number of -OH species on surface, but bidentate carbonate cannot be formed under CO2 adsorptions on its {1 0 0} crystal planes. CO2 participates in this reaction only when it adsorbs on ceria as bidentate carbonate species, so the cube scarcelycatalyzes the reaction. Ceria rods possess sufficient number of -OH groups and bidentate carbonates, leading to the highest catalytic capacity. The dissociated adsorptionof DMC is quite effortless, which means its reversal process, MMC reacts with CH3OH to DMC is the rate-determining step for this whole reaction. The proposed reaction mechanism is illuminated in Fig. 5.

|
Download:
|
| Figure 5. The mechanism of direct synthesis of DMC from methanol and CO2 over different ceria catalysts. | |
4. Conclusions
As a heterogeneous catalyst for DMC formation from carbon dioxide and methanol, ceria with different morphologies shows different reaction activity in the order of rod > octahedron > cube. Analyzed using reaction activity, HR-TEM and in situ FTIR, we hypothesized that the number of -OH groups and different kinds of CO2 adsorptions on ceria surface are decisive factors in this system, which are closely related to ceria crystal planes. Insufficient number of -OH groups on octahedron ceria surface limit its activity. Bidentate carbonates formed on {1 1 0} and {1 1 1} crystal surface is nesessary for the formation of the intermediate product, monodentate methyl carbonate, which leads to the highest activity of ceria rods and lowest activity for ceria cubes. The reaction between MMC with another molecule of methanol to form DMC is believed to be the rate-determining step.
AcknowledgmentsFinancial support by Natural Science Foundation of China (NSFC) (Nos. 21325626, 21176179, U1510203), the Program for New Century Excellent Talents in University (No. NCET-13-0411), the Program of Introducing Talents of Discipline to Universities (No. B06006) is gratefully acknowledged.
| [1] | M. Huang, S. Fabris, CO adsorption and oxidation on ceria surfaces from DFT+U calculations. J. Phys. Chem. C 112 (2008) 8643–8648. DOI:10.1021/jp709898r |
| [2] | A. Aouissi, S.S. Al-Deyab, Comparative study between gas phase and liquid phase for the production of DMC from methanol and CO2. J. Nat. Gas Chem. 21 (2012) 189–193. DOI:10.1016/S1003-9953(11)60353-8 |
| [3] | L. Vivier, D. Duprez, Ceria-based solid catalysts for organic chemistry. Chem. Sus. Chem. 3 (2010) 654–678. DOI:10.1002/cssc.v3:6 |
| [4] | K. Tomishige, T. Sakaihori, Y. Ikeda, K. Fujimoto, A Novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia. Catal. Lett. 58 (1999) 225–229. DOI:10.1023/A:1019098405444 |
| [5] | S. Agarwal, L. Lefferts, B.L. Mojet, Exposed surfaces on shape-controlled ceria nanoparticles revealed through AC-TEM and water-gas shift reactivity. Chem. Sus. Chem. 6 (2013) 1898–1906. DOI:10.1002/cssc.v6.10 |
| [6] | Z.A. Qiao, Z.L. Wu, S. Dai, Shape-controlled ceria-based nanostructures for catalysis applications. Chem. Sus. Chem. 6 (2013) 1821–1833. DOI:10.1002/cssc.v6.10 |
| [7] | D.R. Mullins, The surface chemistry of cerium oxide. Surf. Sci. Rep. 70 (2015) 42–85. DOI:10.1016/j.surfrep.2014.12.001 |
| [8] | Y.J. Guan, E.J.M. Hensen, Y. Liu, Template-free synthesis of sphere, rod and prism morphologies of CeO2 oxidation catalysts. Catal. Lett. 137 (2010) 28–34. DOI:10.1007/s10562-010-0349-5 |
| [9] | H.X. Mai, L.D. Sun, Y.W. Zhang, Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 109 (2005) 24380–24385. DOI:10.1021/jp055584b |
| [10] | K.B. Zhou, X. Wang, X.M. Sun, Q. Peng, Y.D. Li, Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 229 (2005) 206–212. DOI:10.1016/j.jcat.2004.11.004 |
| [11] | X.W. Liu, K.B. Zhou, L. Wang, B.Y. Wang, Y.D. Li, Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 131 (2009) 3140–3141. DOI:10.1021/ja808433d |
| [12] | Y. Yoshida, Y. Arai, S. Kado, K. Kunimori, K. Tomishige, Direct synthesis of organic carbonates from the reaction of CO2 with methanol and ethanol over CeO2 catalysts. Catal. Today 115 (2006) 95–101. DOI:10.1016/j.cattod.2006.02.027 |
| [13] | P. Tundo, M. Selva, The chemistry of dimethyl carbonate. Acc. Chem. Res. 35 (2002) 706–716. DOI:10.1021/ar010076f |
| [14] | K.W. La, M.H. Youn, J.S. Chung, S.H. Baeck, I.K. Song, Synthesis of dimethyl carbonate from methanol and carbon dioxide by heteropolyacid/metal oxide catalysts, in:C.K. Rhee (Ed.), Nanocomposites and Nanoporous Materials VⅡ, Trans Tech Publications Ltd, Stafa-Zurich, 2007, pp. 287-290. |
| [15] | H.J. Hofmann, A. Brandner, P. Claus, Direct synthesis of dimethyl carbonate by carboxylation of methanol on ceria-based mixed oxides. Chem. Eng. Technol. 35 (2012) 2140–2146. DOI:10.1002/ceat.v35.12 |
| [16] | S.B. Xie, A.T. Bell, An in situ raman study of dimethyl carbonate synthesis from carbon dioxide and methanol over zirconia. Catal. Lett. 70 (2000) 137–143. DOI:10.1023/A:1018837317910 |
| [17] | K.T. Jung, A.T. Bell, An in Situ infrared study of dimethyl carbonate synthesis from carbon dioxide and methanol over zirconia. J. Catal. 204 (2001) 339–347. DOI:10.1006/jcat.2001.3411 |
| [18] | M. Aresta, A. Dibenedetto, C. Pastore, Influence of Al2O3 on the performance of CeO2 usd as catalyst in the direct carboxylation of methanol to dimethylcarbonate and the elucidation of the reaction mechanism. J. Catal. 269 (2010) 44–52. DOI:10.1016/j.jcat.2009.10.014 |
| [19] | B.A.V. Santos, C.S.M. Pereira, V.M.T.M. Silva, J.M. Loureiro, A.E. Rodrigues, Kinetic study for the direct synthesis of dimethyl carbonate from methanol and CO2 over CeO2 at high Pressure Conditions. Appl. Catal. A:Gen. 455 (2013) 219–226. DOI:10.1016/j.apcata.2013.02.003 |
| [20] | S.P. Wang, J.J. Zhou, S.Y. Zhao, Y.J. Zhao, X.B. Ma, Enhancements of dimethyl carbonate synthesis from methanol and carbon dioxide:the in situ hydrolysis of 2-cyanopyridine and crystal face effect of ceria. Chin. Chem. Lett. 26 (2015) 1096–1100. DOI:10.1016/j.cclet.2015.05.005 |
| [21] | Z.L. Wu, M.J. Li, D.R. Mullins, S.H. Overbury, Probing the surface sites of CeO2 nanocrystals with well-defined surface planes via methanol adsorption and desorption. Acs Catal. 2 (2012) 2224–2234. DOI:10.1021/cs300467p |
| [22] | S. Rousseau, O. Marie, P. Bazin, Investigation of methanol oxidation over au/catalysts using operando IR spectroscopy:determination of the active sites, intermediate/spectator species, and reaction mechanism, J. Am. Chem. Soc. 132 (2010) 10832–10841. DOI:10.1021/ja1028809 |
| [23] | O. Pozdnyakova, D. Teschner, A. Wootsch, Preferential CO oxidation in hydrogen (PROX) on ceria-supported catalysts, part I:oxidation state and surface species on Pt/CeO2 under reaction conditions. J. Catal. 237 (2006) 1–16. DOI:10.1016/j.jcat.2005.10.014 |
| [24] | Z.L. Wu, A.K.P. Mann, M.J. Li, S.H. Overbury, Spectroscopic investigation of surfacedependent acid-base property of ceria nanoshapes. J. Phys. Chem. C. 119 (2015) 7340–7350. DOI:10.1021/acs.jpcc.5b00859 |
| [25] | C. Binet, M. Daturi, J.C. Lavalley, IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 50 (1999) 207–225. DOI:10.1016/S0920-5861(98)00504-5 |
| [26] | C. Li, Y. Sakata, T. Arai, Carbon monoxide and carbon dioxide adsorption on cerium oxide studied by fourier-transform infrared spectroscopy. Part 1.-formation of carbonate species on dehydroxylated CeO2, at room temperature. J. Chem. Soc. 85 (1989) 929–943. |
| [27] | G.N. Vayssilov, M. Mihaylov, P.S. Petkov, K.I. Hadjiivanov, K.M. Neyman, Reassignment of the vibrational spectra of carbonates, formates, and related surface species on ceria:a combined density functional and infrared spectroscopy investigation. J. Phys. Chem. C 115 (2011) 23435–23454. DOI:10.1021/jp208050a |
| [28] | J. Paier, C. Penschke, J. Sauer, Oxygen defects and surface chemistry of ceria:quantum chemical studies compared to experiment. Chem. Rev. 113 (2013) 3949–3985. DOI:10.1021/cr3004949 |
 2017, Vol. 28
2017, Vol. 28 


