b Fujian Provincial Key Laboratory of Modern Analytical Science and Separation Technology, Minnan Normal University, Zhangzhou 363000, China
Dopamine (DA, 3, 4-dihydroxyphenylethylamine) is one of the naturally occurring catecholamines, which acts as an important neurotransmitters in mammalian brain, and also plays a vital role in the function of human metabolism, hormonal, central nervous, renal, and cardiovascular systems [1]. Abnormal levels of DA in body fluids are the indications of many serious diseases such as Schizophrenia, Huntington’s, Parkinson’s and Alzheimer’s diseases [2]. To date, a great number of instrumental methods have been developed to detect DA, which included capillary electrophoresis, liquid chromatography-tandem mass spectrometry, high performance liquid chromatography, flow injection chemiluminescence and electrochemical analysis [3-6]. Among these techniques, the electrochemical method has attracted much attention because of its advantages of simple operation, cheap instruments, high specificity and rapid analysis. However, the fouling of electrode surface by the oxidized product of DA will result in poor performance at the conventional electrodes, and the other coexisted electroactive substances such as ascorbic acid (AA) and uric acid (UA) will interfere with the detection of DA since they have close oxidation potentials [7]. Therefore, it is still necessary to develop novel approach with better performance.
Graphene is made of carbon atoms organized in a two dimensional (2D) mesh structure [8]. Due to its large surface area, excellent electronic conductivity, and high chemical stability, it shows potential in various fields, such as energy storage, electronic device, biological sensors and so on [8-11]. However, because of the large conjugated effects between the sheet layers, graphene presents poor dispersibility in solution, which limits its application in bioanalysis [5]. The suitable functionalization of graphene can avoid the aggregation and maintain its excellent inherent properties [12]. Non-covalent functionalization through derivation with surfactants [13], organic aromatic molecules [14], or biomacromolecules [15] is a common method. For example, Zhou et al. [13] have prepared CuO-Cu/graphene using sodium dodecyl benzene sulfonate as the dispersing reagent, and applied it for the electrochemical detection of fructose. Chen et al. [16] prepared a noncovalent functionalized graphene solution through the π-π interaction between thionine and graphene. Indeed, noncovalent functionalization method is simple and easy to operate, but the resulting composite materials usually have the disadvantage of low stability due to the weak interactions of non-covalent bonds. In order to overcome such disadvantages, graphene was also modified by means of covalent functionalization methods to improve the dispersibility and stability. Small organic molecules [17] and polymers [18] could be directly introduced to the surface of graphene through surface grafting and chemical reactions. This on the one hand enhances the stability of graphene, and on the other hand, endows the graphene with more new properties through choosing suitable modifiers. Polyaniline (PANI) is one of the most useful conducting polymers due to its facile synthesis, high conductivity, low toxicity, high environmental stability and reversible redox behavior [19, 20]. PANI modified graphene is a very important hybrid material owing the unique properties of excellent environmental stability, ease of preparation and relatively high electrochemical activity [21]. Huang et al. have reported the synthesis of graphene oxide (GO)/PANI nanocomposite with controllable morphologies through in situ polymerization of aniline monomers or GO sheets [22]. Nipapan et al. have prepared a graphene/polyvinylpyrrolidone/PANI-modified electrode for the sensitive amperometric determination of cholesterol in complex human serum sample [23].
Nafion (NF) is a perfluorinated sulphonated cation exchanger with properties of excellent antifouling capacity, chemical inertness and high permeability to cations, and good biocompatibility. Nafion can not only serve as a natural barrier against the interaction of the negatively charged particles, but also prevent the aggregation of graphene. Furthermore, due to the negatively charged Nafion membrane to selectively absorb neutral and positively charged molecules, foreign species such as AA, paracetamol, UA, etc. are easily repelled. For example, Sanghavi et al. had used Nafion as an electrode modifier for organic molecules, and could be a sensitive determination of sumatriptan [24]. Lu et al. also have reported that Nafion had been employed as a protective and selective coating material, and applied in the sensitive and selective determination of glucose [25].
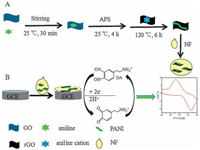
In this work, a highly conductive nanocomposite of PANIreduced graphene oxide (rGO) was prepared using a one-pot solvethermal method, and then the hybrid material of PANI-rGONF was obtained upon ultrasonication. The nanocomposite was characterized by Fourier-transform infrared (FT-IR), Raman spectra, X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Firstly the formation of PANI-rGO hybrids was attributed to the redox reactions between GO and aniline, where the reduction of GO to rGO and polymerization of aniline into PANI occurred simultaneously under the hydrothermal conditions. Then PANI-rGO-NF nanocomposites were prepared by the ultrasonic method. The electrochemical behaviors of the modified electrodes had been carried out using cyclic voltammetry (CV), and we found that PANIrGO-NF/GCE showed good catalytic activity toward the oxidation of DA, and no response to AA and UA, leading to a highperformance DA sensor.
2. Experiment 2.1. Reagents and materialsAA, UA and DA were obtained from Aladdin Co., Ltd. (China). Aniline and graphite powder were purchased from Guangdong Xilong Chemical Co., Ltd (China). Ammonium peroxydisulfate was obtained from Guoyao Chemical Co., Ltd. (China). 5% Nafion was obtained from sigma Co., Ltd (USA). Phosphate buffer solution (PBS) was purchased from Shanghai Kangyi Instrument Co., Ltd. (China). Dopamine hydrochloride injection was supplied by Guangzhou Baiyunshan Mingxing Co., Ltd. (China). Other chemicals used were of analytical reagent grade and obtained commercially. All solvents and chemicals in this work were used without further purification unless specially stated. Doubly distilled water was used throughout experiments.
2.2. Instruments and measurementsX-ray diffraction (XRD; Rigaku, Ultima IV, Japan) were scanned by analyzing the samples using Cu-Kα monochromatic beam at 40 kV and 30 mA with a scan speed of 10°/min ranging from 5° to 90°. Fourier-transform infrared (FTIR) spectra were recorded on a Nicolet iS 10 FT-IR spectrophotometer (Thermo Fisher Scientific Inc., USA). Raman spectra were conducted on an InVia Raman microscope (Renishaw Co. Ltd, UK). Scanning electron microscope (SEM) was performed on an S-4800 electron microscope (Hitachi, Ltd., Japan). Transmission electron microscope (TEM) images were obtained with JEM-2100 TEM (Joel Co., Japan) with an accelerating voltage of 200 kV. All the electrochemical measurements were performed on a CHI 660A electrochemical workstation (Shanghai Chenhua Instruments Co., China) consisting of a conventional three-electrode system: a bare or modified glassy carbon electrode (GCE) as the working electrode (3.0 mm diameter), an Ag/AgCl (saturated KCl) as auxiliary electrode and a Pt wire as counter electrode. All pH measurements were carried out on a Phs-3C exact digital pH meter (Shanghai Mettler-Toledo Instruments Co., China), which was calibrated with standard pH buffers. All experiments were conducted at room temperature.
2.3. Preparation of PANI-rGOGO was synthesized from natural graphite powder using a modified Hummer’s method [26]. The PANI-rGO hybrid was prepared using an in situ chemical oxidative polymerization method. In brief, aniline (2 mg), GO (10 mg) and 0.2 mL of 1 mol/L HCl were added into 17 mL of doubly distilled water with stirring for 30 min at room temperature. After that, ammonium peroxydisulfate (10 mg) was added into the above mixture with continuous stirring for 4 h. Then the suspension was treated with a solvothermal method at 120 ℃ for 6 h. The products were obtained by centrifugation, washing with doubly distilled water, and finally dried in an oven at 60 ℃. Similarly, the control samples of rGO and PANI were prepared following the same procedures.
2.4. Preparation of PANI-rGO modified electrodeTo fabricate the PANI-rGO modified electrode, 1.0 mg PANI-rGO composite was added into 1.0 mL 0.5% Nafion solution, and then ultrasonicated for 2 h to obtain a homogeneous dispersion. The Nafion was utilized as the film-forming reagent and the ionexchange material.
Prior to modification, a GCE was polished with 1.0 μm, 0.3 μm and 0.05 μm alumina powder, and then rinsed ultrasonically with doubly distilled water, ethanol and doubly distilled water for 5 min respectively. Then 5.0 μL of the prepared homogeneous solution (1.0 mg/mL) of PANI-rGO-NF was cast onto the cleaned GCE. After drying in air, the modified electrode was rinsed with doubly distilled water to remove the loosely bound materials, and then the PANI-rGO-NF modified electrode (PANI-rGO-NF/GCE) was obtained (Scheme 1).
|
Download:
|
| Scheme 1. Schematic representation for the preparation of the PANI-rGO-NF. | |
3. Results and discussion 3.1. Structure and morphology characterization
The FTIR spectra of GO, rGO, PANI and PANI-rGO are shown in Fig. 1A. For the GO, the characteristic bands at 1062, 1396, 1622, 1728 and 3450 cm-1 were identified as C-O-C, O-H (C-OH), C5 5C, C=O (-COOH), and O-H (H2O), respectively [21]. These features indicated that graphene oxide was heavily oxidized and consisted of -OH and other oxygen-containing groups. After GO was reduced, the C=O vibration band located at 1728 cm-1 disappeared, but a new shoulder peak located at 1714 cm-1 was observed, suggesting that GO had undergone partial reduction to rGO during the hydrothermal process [27]. For the pure PANI, the peaks at 1570, 1468, 1295, 1105 and 798 cm-1 were attributed to C5 5C stretching of the quinonoid ring, benzenoid ring, the C-N stretching of secondary aromatic, C-H in-plane bending and CH out-of-plane bending modes, respectively [21]. In the PANI-rGO, the peak at 1728 cm-1 (GO) of the C=O stretching vibration was shifted to 1710 cm-1 due to the formation of the amide bond in the hybrid, and the C5 5C stretching intensity ratio of quinonoid ring (1547 cm-1) was improved in the FTIR spectrum of the asprepared composite. It indicated that the structure of the quinonoid ring was promoted and stabilized by the chemical bonds between residual oxygen-contained functional group of the rGO (O5 5C-OH) and amino-group (-NH2) of PANI in the PANI-rGO composite [21].

|
Download:
|
| Figure 1. (A) FTIR spectra of GO (a), rGO (b), PANI (c) and PANI-rGO (d). (B) Raman spectra of Graphite, GO, rGO and PANI-rGO. (C) XRD patterns of Graphite, GO, rGO and PANI-rGO. | |
Fig. 1B showed typical Raman spectra of graphite, GO, rGO and PANI-rGO. As seen, all the four samples showed two characteristic peaks, namely, the G band at approximately 1600 cm-1 assigning to the E2g mode of the C-C bond of the sp2 structure and D band at 1340 cm-1 corresponding to the breathing mode of k-point phonons of A1g symmetry [28]. The graphite showed a strong G band at 1579 cm-1. After oxidation, the G band of GO was broadened and D/G intensity (ID/IG) ratio had been markedly increased. The ID/IG is recognized to reflect the graphitization degree of carbon materials. Through comparison, it could be found that the ID/IG value of PANI-rGO composite (=1.08) was obviously larger than that of GO (=0.97), but comparable to that of rGO (=1.06), confirming that the reduced state of rGO had been formed in the nanocomposite of PANI-rGO [28].
Fig. 1C showed the XRD spectra of graphite, GO, rGO and PANIrGO. The graphite showed very strong peak at 26.6°. For the GO, the introduction of oxygen-containing groups disrupted the carbon hexagonal plane and the interlayer spacing was increased from 0.34 nm to 0.77 nm by weakening the van der Waals forces between the layers [29]. Therefore, the characteristic graphitic plane at 2θ=26.6° shifted to 11.5°. After the reduction to rGO, the peak at 2θ=11.5° disappeared and two new peaks appeared at 17.9° and 24.5°, and the peak was broadened, which indicated that the uncrystallized nature arose due to the poor ordering in the stacking direction [29]. Besides, for the nanocomposite of PANI-rGO, the peak at 2θ=11.5° also disappeared and two peaks at 17.5° and 25° appeared, suggesting that a significant portion of GO was reduced and exfoliated during covalent grafting. Broadening of the peak indicated the amorphous nature of the PANI-rGO due to the poor ordering in the stacking direction [29].
The morphologies of the as-obtained rGO, PANI and PANI-rGO hybrid were further studied by SEM and TEM. The results are shown in Fig. 2. Fig. 2A displayed the SEM image of the rGO film, in which a large area thin film with wrinkle was observed, indicating rGO had been synthesized. The SEM image of PANI showed that the PANI has a meshed uniform fibrous structure (Fig. 2B). From the higher magnification image (inset of Fig. 2B), it could be seen that network contained high pore volume in the microporous structure. When rGO was combined with PANI, some obvious crumpled structure in accordance with the characteristics of rGO were observed, and became much more rough and shaggy compared with rGO sheets, and the sheet was easily folded to form overlapped structures (Fig. 2C). At the same time, the PANI exhibited same morphology as that shown in Fig. 2B, suggesting that the PANI had been coated well with rGO. From the TEM images of rGO (D), PANI (E) and PANI-rGO (F), the well-defined meshedshaped PANI and its combination with rGO could be further confirmed.

|
Download:
|
| Figure 2. SEM image of rGO (A), PANI (B) and PANI-rGO (C). TEM image of rGO (D), PANI (E) and PANI-rGO (F). | |
3.2. Electrochemical behaviors of DA on the modified electrodes
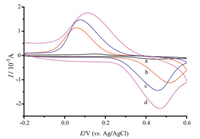
Fig. 3 showed the electrochemical behaviors of 0.05 mmol/L DA on GCE (curve a), PANI-NF/GCE (curve b), rGO-NF/GCE (curve c) and PANI-rGO-NF/GCE (curve d), respectively. As can be seen from Fig. 3a, a pair of weak redox peaks occurred on curve a (Ipa=-1.53 μA, Ipc=0.618 μA). However, the oxidation current (-11.25 μA) was observed to have increased by 6.36 times and the reduction current (11.44 μA) was observed to have increased by 17.51 times on the curve b. On the curve c, the oxidation peak current (-14.4 μA) and the reduction peak current (14.7 μA) were observed to have increased by nearly 8.45 times and 22.77 times, respectively. As expected, on a PANI-rGO/NF composite modified GCE, the oxidation current (-21.9 μA) and the reduction current (17.54 μA) further increased 13.33 times and 27.38 times (curve d). In addition, it was found that the peak-to-peak separation (ΔEp) of DA on PANI-rGO-NF/GCE (0.355 V) was much smaller than those on PANI-NF/GCE (0.475 V) and rGO-NF/GCE (0.383 V), indicating that PANI-rGO-NF/GCE had the best electron-transfer kinetics for the redox of DA. Therefore, it could be concluded that the PANIrGO-NF film was very effective in enhancing the electrochemistry of DA.
|
Download:
|
| Figure 3. CVs of 0.05 mmol/L DA recorded on GCE (a), PANI-NF/GCE (b), rGO-NF/GCE (c) and PANI-rGO-NF/GCE (d) in 0.01 mol/L PBS (pH 5.0) (scan rate: 0.1 V/s). | |
3.3. Electrochemical parameters of DA at PANI-rGO-NF/GCE
In order to better understand the electrochemical process of DA on PANI-rGO-NF/GCE, the electrochemical parameters of DA on the electrode were studied. Fig. 4A showed the CVs of 0.05 mmol/L DA at PANI-rGO-NF/GCE with different scan rates in 0.01 mol/L PBS solution. As seen, with the increase of the scan rate, the redox peaks of DA increased accordingly and the linear relationships of DA between peak currents and the square root of scan rates (υ1/2) were attained as (Fig. 4B): Ipa (10-5 A)=-3.7696υ1/2 (V/s)1/2 + 0.01704, R=0.999; Ipc (10-5 A)=2.908υ1/2 (V/s)1/2 + 0.1848, R=0.994, respectively. This indicated that the reaction process was a diffusion-controlled process. In addition, it was found that the ΔEp of the redox peak enlarged with the increase of the scan rate, which suggested that the electrochemical of DA at the electrode was quasi-reversible. So the electro-transfer kinetic parameters such as the electron transfer coefficient (α) and the standard heterogeneous rate constant (ks) of DA on the PANI-rGO-NF/GCE were calculated according to the following Laviron’s equation [30]:

|
Download:
|
| Figure 4. (A) CV of 0.05 mmol/L DA on PANI-rGO-NF/GCE in 0.01 mol/L PBS (pH 5.0) at various scan rates (a→g: 0.01, 0.04, 0.08, 0.10, 0.15, 0.20, 0.25 V/s), (B) the relationships of peak currents Ip vs. υ1/2, (C) the relationships of peak potentials Ep vs. log υ. | |

|
(1) |
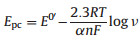
|
(2) |

|
(3) |
where n represents the number of electrons transferred in the reaction, R, T and F have their usual significance.
The linear relationships between the oxidation peak potential (Epa) and reduction peak potential (Epc) with the logarithm values of υ (log υ) were established as Fig. 4C, and linear equations of DA were Epa (V)=0.1247 log υ (V/s) + 0.6023 (R=0.995) and Epc=-0.0804 log υ (V/s) + 0.0599 (R=0.998), respectively. Then the values of a and n were calculated to be 0.47 and 1.89 (≈2), respectively, on the basis of Eqs. (1) and (2). Furthermore, based on Eq. (3), the average value of ks was calculated to be 0.45 s-1, which is larger than those on previously reported electrodes of Au@carbon dots-chitosan/GCE (0.22 s-1) [31] and carbon dots/ chitosan/GCE (0.14 s-1) [32], showing that the sensor in this work has higher catalytic capacity to promote electron transfer kinetics of DA.
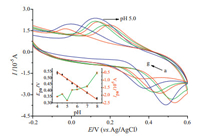
In this work, the effect of pH values on the electrochemical behaviors of DA was also investigated, and the results are showed in Fig. 5. It was found that the redox peak potentials shifted negatively with the increase of pH value from 4.0 to 8.0, indicating that protons were involved in the electrochemical redox process of DA. A good linear relationship between Epa and pH was obtained with linear regression equation of Epa (V)=0.7640 -0.0546 pH (R=0.996) (inset of Fig. 5). And since Ep can be expressed as (at 25 ℃):
|
Download:
|
| Figure 5. CV of 0.05 mmol/L DA on PANI-rGO-NF/GCE in 0.01 mol/L PBS with various pH values (a→g: 4.0, 4.5, 5.0, 5.5, 6.0, 7.0, 8.0). The insets showed the plots of the peak potentials (EP) and peak currents (IP) vs. the pH values of PBS. | |
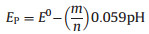
|
(4) |
where E0 is the standard potential, and m the number of protons transferred, the value of m/n was then obtained to be approximately 1 from the slope of the relationship. Therefore, it could be obtained that the redox of DA undergoes a two-electron and twoproton process, which was consistent with the classical electrochemical characteristic of DA [5].
At the same time, the acidity of PBS solution also had a remarkable effect on the peak currents of DA. The insets graph of Fig. 4B showed that the oxidation peak currents (Ipa) increased with the decrease of pH values until it reached 5.0 and then decreased when the pH value is further decreased. Therefore, pH 5.0 of PBS was selected as the optimal acidity in this experiment.
3.4. Electrochemical detection of DAThe DPV responses of 0.01 mol/L PBS (pH 5.0) solution with different concentrations of DA are shown in Fig. 6. It can be seen thattheDPVpeak enhancedwithincreaseofDAconcentration.The values of Ipa are linearly related to the DA concentration over the rangeof 0.05-60.0 mmol/Land 60.0-180.0 mmol/L, withtwolinear regression equation of Ipa (μA)=-0.0473 C (μmol/L) -0.0971 (R=0.996) and Ipa (μA)=-0.0038 C (μmol/L) -2.6608 (R=0.996), respectively. The detection limit of DA could be estimated to be as low as 0.024 μmol/L (3S/N), which was significantly lower than those reported in many previously reported electrochemical sensors (Table 1), which suggested that the developed sensor had the superiority for the highly sensitive determination of DA.

|
Download:
|
| Figure 6. DPVs of the PANI-rGO-NF/GCE in 0.01 mol/L PBS (pH 5.0) with increasing concentrations of DA (a→p: 0, 0.05, 3, 6, 9, 10, 20, 30, 40, 50, 60, 80, 100, 120, 150, 180 μmol/L). The insets show the plots of the peak current (Ipa) vs. the concentrations of DA (C). | |
|
|
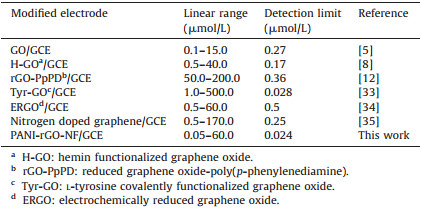
Table 1 Comparison of the proposed electrode to other modified electrodes for DA determination. |
3.5. Anti-interference capacity
It is well known that AA and UA often coexist with DA in the biological systems and their concentrations are usually much higher than that of DA. Therefore, it is very necessary to study their interference for the detection of DA. Fig. 7A depicted the responses of the PANI-rGO-NF/GCE in a blank solution, 6 mmol/L AA solution, 0.05 mM DA solution and the mixture solution of 0.05 mmol/L DA and 6 mM AA, respectively. It was clear that when blank solution of PBS or the PBS in the presence of AA was tested, the redox response was observed on PANI-rGO-NF/GCE, suggesting that the AA is electroactive under the tested conditions. However, when the mixture of 0.05 mmol/L DA and 6 mmol/L AA was determined by PANI-rGO-NF/GCE, it was found that a pair of clear redox peaks appeared, and this pair of redox peaks was very similar to that of the solution with only 0.05 mmol/L DA. This result suggested that DA could be selectivity detected in the presence of excess of AA, and the signal intensity could not be affected by the AA. Also, the influence of UA on the electrochemical signal of DA on the PANIrGO-NF/GCE was investigated, and the obtained CVs were displayed in Fig. 7B. The results indicated that UA also had not electrochemical signal on PANI-rGO-NF/GCE, and it had not effect on the electrochemical response of DA.

|
Download:
|
| Figure 7. (A) CV of PANI-rGO-NF/GCE in 0.01 mol/L pH 5.0 PBS with blank solution without and with 6 mmol/L AA solution, or 0.05 mmol/L DA solution, or the mixture of 0.05 mmol/L DA and 6 mmol/L AA. (B) CV of PANI-rGO-NF/GCE in 0.01 mol/L pH 5.0 PBS blank solution without and with 5 mmol/L UA solution or 0.05 mmol/L DA solution or the mixture of 0.05 mmol/L DA and 5 mmol/L UA (scan rates: 0.1 V/s). | |
Furthermore, the influences from the other common coexisting substances were also investigated. When the relative error (Er) exceeded 5%, each matter was considered as an interfering agent. It was found that most ions and common substances at high concentration only caused negligible changes ( < 5%): Na+, K+, Cl-, NO3-, SO42- (500-fold), Ca2+, Zn2+, Mg2+, Pb2+ (150-fold), lysine, threonine, cysteine, tryptophan and glucose (150 fold), The results indicated that high selectivity and high activity toward the oxidation of DA at the PANI-rGO-NF/GCE were achieved.
3.6. Repeatability and stability of the PANI-rGO-NF/GCEThe repeatability of PANI-rGO-NF/GCE was also investigated by detecting the response to 0.05 mmol/L DA for six different modified electrodes. The RSD of the anodic peak current was found to be 1.4%, and this indicated that the modified electrodes had acceptable repeatability. Additionally, the stability of the modified electrode was also estimated, and the DA sample was determined every 5 days. After measurement, the modified electrode was cleaned with PBS (pH 5.0) to eliminate the adsorption and stored in a refrigerator at 4 ℃, and the anodic peak current of 0.05 mmol/L DA lost only 5.8% of the initial response after 25 days. These results indicated that the PANI-rGONF/GCE has good repeatability and stability, and the sensor can be used for repetitive determinations over at least 25 days.
3.7. Real sampleTo evaluate feasibility of the proposed method for DA determination in real samples, the modified electrode was used to detect DA in dopamine hydrochloride injection. The five parallel experiments were performed and recovery tests were conducted using the standard addition method. The results are listed in Table 2. The obtained recoveries ranged from 96.24% to 107.61%, thereby validating the good recovery and practicability of the developed sensor.
|
|
Table 2 Determination of DA in dopamine hydrochloride injections (n=5). |
4. Conclusions
In summary, PANI-rGO composites have been successfully prepared by the heat treatment of GO and aniline, and the PANIrGO-NF nanocomposites were produced by the ultrasonic method. Most importantly, the PANI-rGO-NF composites exhibited good catalytic activity toward the oxidation of DA, and no response to AA and UA which had concentration of one hundred times higher than that of the DA. The peak currents are linearly correlated with the concentration of DA in the range from 0.05 μmol/L to 60.0 μmol/L (R=0.996) and 60.0 μmol/L to 180.0 μmol/L (R=0.996) with a detection limit of 0.024 μmol/L (S/N=3). The electrochemical parameters such as the electron transfer rate constant, diffusion coefficient, and electron/proton transfer number of DA on PANIrGO-NF/GCE were also studied. In addition, the modified electrode exhibited excellent reproducibility and stability.
AcknowledgmentsThis research work was financially supported by the National Natural Science Foundation of China (Nos. 21275127 and 21301143), Natural Science Foundation of Fujian Province (No. 2011J01059) and Key Provincial University Project of Fujian (No. JK2011032).
| [1] | S. Liu, X.R. Xing, J.H. Yu, A novel label-free electrochemical aptasensor based on graphene-polyaniline composite film for dopamine determination. Biosens. Bioelectron. 36 (2012) 186–191. DOI:10.1016/j.bios.2012.04.011 |
| [2] | X.W. Yu, K.X. Sheng, G.Q. Shi, A three-dimensional interpenetrating electrode of reduced graphene oxide for selective detection of dopamine. Analyst 139 (2014) 4525–4531. DOI:10.1039/C4AN00604F |
| [3] | H.M. Huang, C.H. Lin, Methanol plug assisted sweeping-micellar electrokinetic chromatography for the determination of dopamine in urine by violet light emitting diode-induced fluorescence detection. J. Chromatogr. B 816 (2005) 113–119. DOI:10.1016/j.jchromb.2004.11.018 |
| [4] | G.Y. Fan, W.J. Huang, Synthesis of ruthenium/reduced graphene oxide composites and application for the selective hydrogenation of halonitroaromatics. Chin. Chem. Lett. 25 (2014) 359–363. DOI:10.1016/j.cclet.2013.11.044 |
| [5] | F. Gao, X.L. Cai, X. Wang, Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sensor. Actuators B 186 (2013) 380–387. DOI:10.1016/j.snb.2013.06.020 |
| [6] | Y.S. Zhao, S.L. Zhao, J.M. Huang, F.G. Ye, Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 85 (2011) 2650–2654. DOI:10.1016/j.talanta.2011.08.032 |
| [7] | Y.R. Kim, S. Bong, Y.J. Kang, Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 25 (2010) 2366–2369. DOI:10.1016/j.bios.2010.02.031 |
| [8] | H.L. Zou, B.L. Li, H.Q. Luo, N.B. Li, A novel electrochemical biosensor based on hemin functionalized graphene oxide sheets for simultaneous determination of ascorbic acid, dopamine and uric acid. Sens. Actuators B 207 (2015) 535–541. DOI:10.1016/j.snb.2014.10.121 |
| [9] | F.Y. Zhang, Y.J. Li, Y.E. Gu, Z.H. Wang, C.M. Wang, One-pot solvothermal synthesis of a Cu2O/graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim. Acta 173 (2011) 103–109. DOI:10.1007/s00604-010-0535-6 |
| [10] | Y.L. Du, X. Gao, X.L. Ye, Composition and architecture-engineered AuSnO2/GNs-SWCNTs nanocomposites as ultrasensitive and robust electrochemical sensor for antioxidant additives in foods. Sens. Actuators B 203 (2014) 926–934. DOI:10.1016/j.snb.2014.06.094 |
| [11] | X.J. Zhao, X.H. Xia, S.Q. Yu, C.M. Wang, An electrochemical sensor for honokiol based on a glassy carbon electrode modified with MoS2/graphene nanohybrid film. Anal. Methods 6 (2014) 9375–9382. DOI:10.1039/C4AY01790K |
| [12] | S. Liu, B. Yu, T. Zhang, Preparation of crumpled reduced graphene oxide-poly (pphenylenediamine) hybrids for the detection of dopamine. J. Mater. Chem. A 1 (2013) 13314–13320. DOI:10.1039/c3ta12594g |
| [13] | S.H. Zhou, D.L. Wei, H.Y. Shi, Sodium dodecyl benzene sulfonate functionalized graphene for confined electrochemical growth of metal/oxide nanocomposites for sensing application. Talanta 107 (2013) 349–355. DOI:10.1016/j.talanta.2013.01.041 |
| [14] | Q. Su, S.P. Pang, V. Alijani, Composites of graphene with large aromatic molecules. Adv. Mater. 21 (2009) 3191–3195. DOI:10.1002/adma.v21:31 |
| [15] | T. Premkumar, K.E. Geckeler, Graphene-DNA hybrid materials:assembly, applications, and prospects, Prog. Polym. Sci. 37 (2012) 515–529. |
| [16] | C. Chen, W.T. Zhai, D.D. Lu, H.B. Zhang, W.G. Zheng, A facile method to prepare stable noncovalent functionalized graphene solution by using thionine. Mater. Res. Bull. 46 (2011) 583–587. DOI:10.1016/j.materresbull.2010.12.024 |
| [17] | S. Stankovich, R.D. Piner, S.T. Nguyen, R.S. Ruoff, Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 44 (2006) 3342–3347. DOI:10.1016/j.carbon.2006.06.004 |
| [18] | Y. Lin, J. Jin, M. Song, Preparation and characterisation of covalent polymer functionalized graphene oxide. J. Mater. Chem. 21 (2011) 3455–3461. DOI:10.1039/C0JM01859G |
| [19] | D. Li, J.X. Huang, R.B. Kaner, Polyaniline nanofibers:a unique polymer nanostructure for versatile applications. Acc. Chem. Res. 42 (2009) 135–145. DOI:10.1021/ar800080n |
| [20] | H. Zhou, Y.P. Sun, G. Li, S.J. Chen, Y. Lu, Interfacial assembly and electrochemical properties of nafion-modified-graphene/polyaniline hollow spheres. Polymer 55 (2014) 4459–4467. DOI:10.1016/j.polymer.2014.06.079 |
| [21] | Y.H. Jin, M. Fang, M.Q. Jia, In situ one-pot synthesis of graphene-polyaniline nanofiber composite for high-performance electrochemical capacitors. Appl. Surf. Sci. 308 (2014) 333–340. DOI:10.1016/j.apsusc.2014.04.168 |
| [22] | Y.F. Huang, C.W. Lin, Facile synthesis and morphology control of graphene oxide/polyaniline nanocomposites via in-situ polymerization process. Polymer 53 (2012) 2574–2582. DOI:10.1016/j.polymer.2012.04.022 |
| [23] | N. Ruecha, R. Rangkupan, N. Rodthongkum, O. Chailapakul, Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosens. Bioelectron. 52 (2014) 13–19. DOI:10.1016/j.bios.2013.08.018 |
| [24] | S. Liu, L. Wang, Y.L. Luo, Polyaniline nanofibres for fluorescent nucleic acid detection. Nanoscale 3 (2011) 967–969. DOI:10.1039/c0nr00873g |
| [25] | P. Lu, J. Yu, Y.T. Lei, Synthesis and characterization of nickel oxide hollow spheres-reduced graphene oxide-nafion composite and its biosensing for glucose. Sensor. Actuators B 208 (2015) 90–98. DOI:10.1016/j.snb.2014.10.140 |
| [26] | W.S. Hummers Jr, R.E. Offeman, Preparation of graphitic oxide. J. Am. Chem. Soc. 80 (1958) 1339. DOI:10.1021/ja01539a017 |
| [27] | X.L. Huang, N.T. Hu, R.G. Gao, Reduced graphene oxide-polyaniline hybrid:preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 22 (2012) 22488–22495. DOI:10.1039/c2jm34340a |
| [28] | Y.Z. Yang, F. Gao, X.L. Cai, β-Cyclodextrin functionalized graphene as a highly conductive and multi-site platform for DNA immobilization and ultrasensitive sensing detection. Biosens. Bioelectron. 74 (2015) 447–453. DOI:10.1016/j.bios.2015.06.018 |
| [29] | R. Thekkayil, H. John, P. Gopinath, Grafting of self assembled polyaniline nanorods on reduced graphene oxide for nonlinear optical application. Synth. Met. 185-186 (2013) 38–44. DOI:10.1016/j.synthmet.2013.09.035 |
| [30] | E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interf. Electrochem. 101 (1979) 19–28. DOI:10.1016/S0022-0728(79)80075-3 |
| [31] | Q.T. Huang, H.Q. Zhang, S.R. Hu, A sensitive and reliable dopamine biosensor was developed based on the Au@carbon dots-chitosan composite film. Biosens. Bioelectron. 52 (2014) 277–280. DOI:10.1016/j.bios.2013.09.003 |
| [32] | Q.T. Huang, S.R. Hu, H.Q. Zhang, Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine. Analyst 138 (2013) 5417–5423. DOI:10.1039/c3an00510k |
| [33] | X.H. Wang, F.F. Zhang, J.F. Xia, Modification of electrode surface with covalently functionalized graphene oxide by L-tyrosine for determination of dopamine. J. Electroanal. Chem. 738 (2015) 203–208. DOI:10.1016/j.jelechem.2014.12.005 |
| [34] | L. Yang, D. Liu, J.S. Huang, T.Y. You, Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B 193 (2014) 166–172. DOI:10.1016/j.snb.2013.11.104 |
| [35] | Z.H. Sheng, X.Q. Zheng, J.Y. Xu, Electrochemical sensor based on nitrogen doped graphene:simultaneous determinationof ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 34 (2012) 125–131. DOI:10.1016/j.bios.2012.01.030 |
 2017, Vol. 28
2017, Vol. 28