b Department of Natural Product Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China
Artocarpus, belonging to the mulberry family Moraceae, is a genus of approximately 60 trees and shrubs distributed in the Southeast Asian and Pacific regions. A. heterophyllus Lam, popularly known as jackfruit, is one of the important trees in the home gardens of tropical India, Bangladesh, Nepal, Sri Lanka, Vietnam, Thailand, Malaysia, Indonesia, Philippines, Africa, and Brazil [1]. It is also an important fruit in southern China, distributed and cultivated in Hainan, Guangdong, Guangxi, and Yunnan provinces [2]. Different parts of this species have been used for medicinal purposes, such as alleviating asthma and fever (the roots), relieving biliousness and diarrhea (the seeds), acting as a sedative for convulsion (the wood), stimulating lactation in women and animals and acting as an antisyphilitic and vermifuge in humans (the leaves), and relieving ulcers and wounds (the leaf ash) [3, 4]. Wood and bark of this plant are rich sources of prenylated flavonoids, stilbenoids, triterpenoids, and steroids [1]. Some of these compounds have exhibited interesting biological activities, such as cytotoxicity [5, 6], antioxidative activity [7], anti-inflammatory activity [8], antimalarial activity [9], inhibition of tyrosinase and melanin biosynthesis [10, 11], and inhibition of 5areductase [12]. In our previous study, 10 chalcones, 8 flavones, and one 2-arylbenzofuran derivative were isolated from the twigs of A. heterophyllus, among which some showed moderate inhibitory activity on the proliferation of the PC-3 and H460 cell lines [6]. In the course of our ongoing study, the 95% EtOH extract of the leaves of A. heterophyllus was investigated and one new 2-arylbenzofuran derivative, artocarstilbene B (1), one new benzaldehyde derivative, (E)-3, 5-dihydroxy-4-(3-methylbut-1-enyl) benzaldehyde (2), as well as 18 known ones (3-20) (Fig. 1) were obtained. Their structures were elucidated on the basis of extensive spectroscopic techniques including 1D and 2D NMR and HR-ESIMS. Herein, we report the structure elucidation as well as the anti-proliferative activity of these compounds to the PC-3 (human prostate cancer), NCI-H460 (human lung cancer), and A549 (human lung adenocarcinoma epithelial) cells.

|
Download:
|
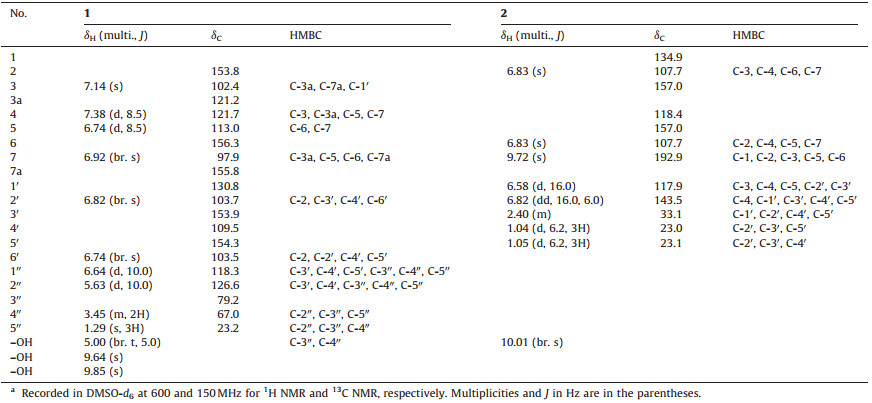
| Figure 1. Structure of compounds 1 and 2. | |
2. Experimental 2.1. General
MTT was purchased from Sigma Chemical Co., (St. Louis, MO, USA). Doxorubicin was purchased from Shenzhen Main Luck Pharmaceuticals Inc. (Shenzhen, China). Optical rotations were measured on a Perkin-Elmer 241 MC polarimeter. IR spectra were recorded on a Nicolet iN 10 Micro FTIR spectrometer by transmission mode. UV spectra were obtained on a Shimadzu UV-2550 spectrophotometer. NMR spectra were measured on a Bruker Avance DRX-600 spectrometer operating at 600 (1H) and 150 (13C) MHz with TMS as internal standard. HR-ESIMS were carried out on a LTQ-Orbitrap XL mass spectrometer. HPLC was performed on an Agilent 1260 HPLC system equipped with a G1311C isopump, a G1315D DAD detector, and a YMC-Pack ODS-A column (10 mm × 250 mm, 5 μm). All solvents used were of analytical grade (Laiyang Chemical Reagent Co., Ltd., Shandong, China). Silica gel (200-300 mesh; Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), C18 reversed-phase silica gel (YMC ODS-A gel, YMC Co., Ltd.), MCI-gel (CHP20P, 75-150 μm, Mitsubishi Chemical Industries Ltd.), and Sephadex LH-20 (Pharmacia Biotek, Denmark) were used for column chromatography (CC). Thin layer chromatography (TLC) was carried out with high-performance TLC plates precoated with silica gel GF254 (Qingdao Haiyang Chemical Co., Ltd.). Spots of TLC were observed under UV or visualized by spraying with H2SO4-EtOH (1:9) followed by heating.
2.2. Plant materialThe leaves of A. heterophyllus were collected from Mengla County, Yunnan Province, China, in July 2009. The plant material was identified by Dr. Tao Shen, Shandong University. A voucher specimen (AH02-2009-07) was deposited at the Department of Natural Products Chemistry, School of Pharmaceutical Sciences, Shandong University.
2.3. Extraction and isolationThe leaves of A. heterophyllus (5.0 kg), air-dried and powdered, were percolated with 95% EtOH (3× 25 L, each for one week) at room temperature. The combined extracts were concentrated under reduced pressure to afford a dark gum (123.8 g), which was suspended in warm water and partitioned successively with petroleum ether (4× 1.2 L) and EtOAc (4× 1.2 L). The EtOAc soluble part (57.3 g) was subjected to CC of silica gel eluted with petroleum ether-EtOAc (20:1→1:2) to yield 12 fractions (Fr. 1-Fr. 12). Fr. 4 (3.2 g) was crystallized to yield 19 (150.1 mg). Fr. 5 (7.7 g) was subjected to CC of MCI (30%→100% MeOH) to obtain five subfractions (Fr. 5.1-Fr. 5.5). Fr. 5.2 (820.0 mg) was further separated by CC of Sephadex LH-20 (MeOH) and preparative HPLC (MeOH-H2O, 88:12, 1.8 mL/min) to afford 16 (9.5 mg, tR=10.61 min), 7 (9.0 mg, tR=12.31 min), and 2 (1.2 mg, tR=15.02 min). Fr. 6 (6.3 g) was subjected to CC of MCI (30%→100% MeOH) to obtain six subfractions (Fr. 6.1-Fr. 6.6). Fr. 6.2 (1.1 g) was further separated by CC of Sephadex LH-20 (MeOH) and preparative HPLC (MeOH-H2O, 80:20, 1.8 mL/min) to afford 12 (3.0 mg, tR=8.06 min) and 14 (20.9 mg, tR=10.02 min). Fr. 6.3 (0.9 g) was first separated by CC of silica gel (petroleum ether-acetone, 10:1) to obtain the major part, which was purified by CC of Sephadex LH-20 (MeOH) to afford 13 (1.6 mg). Fr. 6.6 (1.3 g) was first separated by CC of silica gel (petroleum etheracetone, 10:1) to obtain the major part, which was then separated by CC of C18 reversed-phase silica gel (30%→100% MeOH) to obtain 4 (9.5 mg) and 8 (3.0 mg). Fr. 7 (10.3 g) was subjected to CC of MCI (30%→100% MeOH) to obtain seven subfractions (Fr. 7.1-Fr. 7.7). Fr. 7.1 (0.9 g) was further purified by CC of Sephadex LH-20 (MeOH) and HPLC (MeOH-H2O, 78:22, 1.8 mL/min) to yield 15 (20.9 mg, tR=13.61 min). Fr. 7.4 was separated by Sephadex LH-20 CC (MeOH) to obtain the major part, which was then separated by HPLC (MeOH-H2O, 78:22, 1.8 mL/min) to obtain 3 (7.6 mg, tR=15.90 min) and 5 (6.3 mg, tR=21.82 min). Fr. 8 (6.1 g) was subjected to CC of MCI (30%→100% MeOH) to obtain six subfractions (Fr. 8.1-Fr. 8.6). Fr. 8.1 (0.7 g) was separated by CC of Sephadex LH-20 (MeOH) and CC of C18 reversed-phase silica gel (30%→100% MeOH) to obtain 9 (4.3 mg) and 11 (6.2 mg). Fr. 8.2 (0.9 g) was separated by preparative HPLC (MeOH-H2O, 75:25, 1.8 mL/min) to yield 17 (6.0 mg, tR=19.50 min), 1 (4.0 mg, tR=20.85 min), 10 (8.1 mg, tR=22.02 min), and 6 (5.2 mg, tR=24.02 min). Fr. 9 (2.3 g) was separated by CC of MCI (30%→100% MeOH) to obtain four subfractions (Fr. 9.1-Fr. 9.4). Fr. 9.2 (0.9 g) was separated by CC of C18 reversed-phase silica gel (30%→100% MeOH) to obtain 18 (8.9 mg). Fr. 9.3 was crystallized to yield 20 (5.0 mg).
Artocarstilbene B (1). Yellowish oil. IR (KBr)υmax: 3265, 2928, 1617, 1564, 1489, 1426, 1365, 1148, 971, 823 cm-1. UV (MeOH) λmax (log ε): 226 (4.03) nm. 1H NMR and 13C NMR data, see Table 1. (-)-HR-ESIMS: m/z 323.0917 [M-H]- (Calcd. for C19H15O5, 323.0919).
|
|
Table 1 1H NMR and 13C NMR data and detailed HMBC correlations for compounds 1 and 2.a |
(E)-3, 5-Dihydroxy-4-(3-methylbut-1-enyl) benzaldehyde (2). Yellowish oil. IR (KBr) υmax: 3298, 2960, 2929, 1684, 1514, 1437, 1391, 1338, 1206, 1042, 842 cm-1. UV (MeOH) λmax (log e): 203 (3.77) nm. 1H NMR and 13C NMR data, see Table 1. (-)-HRESIMS: m/z 205.0869 [M-H]- (Calcd. for C12H13O3, 205.0865).
2.4. Cell lines and cell culturePC-3 cells (ATCC CRL-1435 human prostate adenocarcinoma), NCI-H460 cells (ATCC HTB 177 human lung carcinoma), and A549 cells (ATCC CCL-185 human lung adenocarcinoma epithelial) were cultured in RPMI-1640 medium (HyClone, Thermo Fisher Scientific Inc., Waltham, MA, USA). The medium was supplemented with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, Carlsbad, CA, USA), 100 μg/mL penicillin and 100 μg/mL streptomycin. Cells were cultured in a humidified atmosphere of 5% CO2 at 37 ℃.
2.5. Cytotoxicity assayThe tetrazolium-based colorometric assay (MTT assay) was used to determine cell viability. The cancer cells were all cultured under standard culture conditions. The test compounds (1-20) or vehicle control (dimethyl sulfoxide, DMSO) were added to appropriate wells and the cells were incubated for 72 h. Then MTT solution was added into the assay plates (final concentration, 0.5 mg/mL). After shaking for 10 s, plates were returned to incubator and kept for 4 h. The supernatants were removed carefully, followed by the addition of 100 μL of DMSO to each well to dissolve the precipitate. Then, the absorbance was measured at 570 nm with a Model 680 microplate reader (Bio-Rad, Hercules, CA, USA). The percent viability was expressed as absorbance in the presence of test compound as a percentage of that in the vehicle control. Doxorubicin and DMSO were used as positive and negative controls.
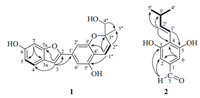
3. Results and discussionCompound 1 was obtained as a yellowish oil. The highresolution ESI-MS showed the pseudo molecular ion [M-H]- peak at m/z 323.0917, corresponding to the molecular formula C19H16O5 (Calcd. for C19H16O5, 323.0919). The IR spectrum displayed absorption bands for hydroxyl (3265 cm-1) and benzene ring (1684 cm-1 and 1564 cm-1). The 1H NMR (Table 1) showed resonances for one 1, 3, 4, 5-tetrasubstituted benzene ring (δH 6.82, br. s and 6.74, br. s), one substituted benzofuran ring (δH 7.14, s, H-3; 7.38, d, J=8.5 Hz, H-4; 6.74, d, J=8.5 Hz, H-5; and 6.92, br. s, H-7), one double bond (δH 6.64, d, J=10.0 Hz and 5.63, d, J=10.0 Hz), one methyl singlet (δH 1.29, s, 3H), and one oxygenated methylene (δH 3.45, m, 2H). The 13C NMR showed resonances for 19 carbons, which included one methyl, one oxygenated methylene, eight methine (all sp2), and nine quaternary (one sp3 and eight sp2) carbons as discerned from the HSQC spectrum. The NMR data (Table 1) for 1 was very similar to those of moracin D (16) [13]. The only difference was absence of one methyl in 16 and instead, one oxygenated methylene (δH 3.45, m, 2H; δC 67.0) was observed in 1, indicating oxidation of a methyl to a hydroxymethyl in 1. In the HMBC of 1 (Fig. 2), the methylene protons were observed to correlated with C-5" (δC 23.2), C-3" (δC 79.2), and C-2" (δC 126.6), which also supported the above conclusion. Thus, compound 1 was determined as 2-[5-hydroxy-(2-hydroxymethyl-2-methyl) benzopyran]-1-benzofuran-5-ol, and named artocarstilbene B.
|
Download:
|
| Figure 2. Key HMBC (H→C) and 1H-1H COSY (-) correlations of 1 and 2. | |
Compound 2 was also obtained as a yellowish oil. The molecular formula was deduced as C12H14O3 from the HRESIMS of 2, which showed a pseudo molecular ion [M-H]- at m/z 205.0869 (Calcd. for C12H13O3, 205.0865). The IR spectrum showed absorption bands for hydroxy (3298 cm-1) and benzene (1684 and 1514 cm-1) groups. The 1H NMR (Table 1) showed signals for an aldehyde (δH 9.72, s), a symmetrical 1, 2, 3, 5-tetrasubstituted benzene ring (δH 6.83, s, H-2 and H-6), a double bond (δH 6.58, d, J=16.0 Hz and 6.82, dd, J=16.0, 6.0 Hz), and one isopropyl (δH 2.40, m; 1.04 and 1.05, each d, J=6.2 Hz, each 3H). The 13C NMR showed signals for 12 carbons, including a symmetrically substituted benzene ring at δC 134.9 (C-1), 107.7 (C-2, 6), 157.0 (C-3, 5), and 118.4 (C-4), as well as an aldehyde (δC 192.9) and a double bond (δC 117.9 and 143.5). The above information indicated that compound 2 was a substituted benzaldehyde. In the HMBC of 2 (Fig. 2), correlations of H-1'/C-3(C-5); H-2'/C-4, C-3', and C-5'; and H-7(δH 9.72)/C-1, C-2, and C-3 verified the structure of 2 to be (E)-3, 5-dihydroxy-4-(3-methylbut-1-enyl) benzaldehyde.
The known compounds were identified by comparison of their 1H NMR, 13C NMR and MS data with those reported in the literatures as one chalcone, artocarmitin B (3) [14], five flavones, artocarpin (4) [15], cudraflavone C (5) [16], albanin A (6) [17], cudraflavone (7) [18], and brosimone I (8) [18], two flavanones, norartocarpanone (9) [19] and euchrenone a7 (10) [20], six 2-arylbenzofuran derivatives, moracin M (11) [21], moracin C (12) [13], albafuran B (13) [22], artoindonesianin B-1 (14) [23], demethylmoracin I (15) [24], and moracin D (16) [13], one stilbenoid, artocarbene (17) [25], one lignan, 2, 6, 20, 60-tetramethoxy-4, 4'-bis (2, 3-epoxy-1-hydroxy-propyl) biphenyl (18) [26], one triterpenoid, griffithine A (19) [27], and one steroid, 5α, 6α-epoxy-24(R)-methylcholesta-7, 22-dien-3β-ol (20) [28] (Fig.S1 in Supporting information).
Compounds 1-20 were evaluated for the in vitro inhibition of cell proliferation against the PC-3, NCI-H460, and A549 cancer cell lines using the MTT method. Compounds with IC50 value < 50 μmol/L were listed in Table 2. In our previous study [6], moderate to weak cytotoxicity was found for compounds 3-5 against the PC-3 and NCI-H460 cells, and in this report they showed similar results. Besides this, 3-5 also exhibited cytotoxicity against the A549 cells. Compounds 10, 14, and 16 also showed weak inhibitory activity (IC50 < 20 μmol/L) to some or all of the three cancer cell lines. The other compounds were inactive in our study (IC50 > 20 μmol/L).
|
|
Table 2 Cytotoxicity of some compounds against human cancer cells. |
4. Conclusion
In summary, 20 compounds including one new 2-arylbenzofuran derivative, artocarstilbene B (1) and one new benzaldehyde derivative, (E)-3, 5-dihydroxy-4-(3-methylbut-1-enyl) benzaldehyde (2) were isolated from the leaves of A. heterophyllus. Compounds 3-5, 10, 14, and 16 showed inhibitory activity against the proliferation of the PC-3, NCI-H460, and/or A549 cancer cell lines.
AcknowledgmentsFinancial supports from National Natural Science Foundation of China (No. 31500280) and Shandong Provincial Natural Science Foundation, China (No. ZR2015PC006) are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.024.
| [1] | M.S. Baliga, A.R. Shivashankara, R. Haniadka, J. Dsouza, H.P. Bhat, Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit):a review. Food Res. Int. 44 (2011) 1800–1811. DOI:10.1016/j.foodres.2011.02.035 |
| [2] | Y.Z. Li, Q. Mao, F. Feng, C.H. Ye, Genetic diversity within a jackfruit (Artocarpus heterophyllus Lam.) germplasm collection in China using AFLP markers. Agric. Sci. China 9 (2010) 1263–1270. DOI:10.1016/S1671-2927(09)60215-7 |
| [3] | J.G.S. Maia, E.H.A. Andrade, M. das Graças, B. Zoghbi, Aroma volatiles from two fruit varieties of jackfruit (Artocarpus heterophyllus Lam.). Food Chem 85 (2014) 195–197. |
| [4] | M.R. Khan, A.D. Omoloso, M. Kihara, Antibacterial activity of Artocarpus heterophyllus. Fitoterapia 74 (2003) 501–505. DOI:10.1016/S0367-326X(03)00120-5 |
| [5] | T. Nomura, Y. Hano, M. Aida, Isoprenoid-substituted flavonoids from Artocarpus plants (Moraceae). Heterocycles 47 (1998) 1179–1205. DOI:10.3987/REV-97-SR(N)9 |
| [6] | X.X. Di, S.Q. Wang, B. Wang, New phenolic compounds from the twigs of Artocarpus heterophyllus. Drug Discov. Ther. 7 (2013) 24–28. |
| [7] | U.B. Jagtap, S.N. Panaskar, V.A. Bapat, Evaluation of antioxidant capacity and phenol content in jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Foods Hum. Nutr 65 (2010) 99–104. DOI:10.1007/s11130-010-0155-7 |
| [8] | B.L. Wei, J.R. Weng, P.H. Chiu, Antiinflammatory flavonoids from Artocarpus heterophyllus and Artocarpus communis. J. Agric. Food Chem. 53 (2005) 3867–3871. DOI:10.1021/jf047873n |
| [9] | C. Boonlaksiri, W. Oonanant, P. Kongsaeree, An antimalarial stilbene from Artocarpus integer. Phytochemistry 54 (2000) 415–417. DOI:10.1016/S0031-9422(00)00074-1 |
| [10] | E.T. Arung, K. Shimizu, R. Kondo, Structure-activity relationship of prenyl-substituted polyphenols from Artocarpus heterophyllus as inhibitors of melanin biosynthesis in cultured melanoma cells. Chem. Biodivers. 4 (2007) 2166–2171. DOI:10.1002/(ISSN)1612-1880 |
| [11] | K. Shimizu, R. Kondo, K. Sakai, S.H. Lee, H. Sato, The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med. 64 (1998) 408–412. DOI:10.1055/s-2006-957470 |
| [12] | K. Shimizu, M. Fukuda, R. Kondo, K. Sakai, The 5ǁ-reductase inhibitory components from heartwood of Artocarpus incisus:structure-activity investigations. Planta Med. 66 (2000) 16–19. DOI:10.1055/s-2000-11114 |
| [13] | M. Takasugi, S. Nagao, S. Ueno, Moracin C and D, new phytoalexins from diseased mulberry. Chem. Lett. 7 (1978) 1239–1240. DOI:10.1246/cl.1978.1239 |
| [14] | N.T. Nguyen, M.H.K. Nguyen, H.X. Nguyen, N.K.N. Bui, M.T.T. Nguyen, Tyrosinase inhibitors from the wood of Artocarpus heterophyllus. J. Nat. Prod. 75 (2012) 1951–1955. DOI:10.1021/np300576w |
| [15] | Y.H. Wang, A.J. Hou, L. Chen, New isoprenylated flavones, artochamins A-E, and cytotoxic principles from Artocarpus chama. J. Nat. Prod. 67 (2004) 757–761. DOI:10.1021/np030467y |
| [16] | Y. Hano, Y. Matsumoto, K. Shinohara, J.Y. Sun, T. Nomura, Cudraflavones C and D, two new prenylflavones from the root bark of Cudrania ricuspidata (carr.) bur. Heterocycles 31 (1990) 1339–1344. DOI:10.3987/COM-90-5416 |
| [17] | F. Ferrari, I. Messana, M.d.C.M. de Araujo, Structures of three new flavone derivatives, brosimones G, H, and I, from Brosimopsis oblongifolia. Planta Med. 55 (1989) 70–72. DOI:10.1055/s-2006-961830 |
| [18] | Z.P. Zheng, K.W. Cheng, J.T.K. To, H.T. Li, M.F. Wang, Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Mol. Nutr. Food Res. 52 (2008) 1530–1538. DOI:10.1002/mnfr.v52:12 |
| [19] | N. Jun, G. Hong, K. Jun, Synthesis and evaluation of 2', 4', 6'-trihydroxychalcones as a new class of tyrosinase inhibitors. Bioorg. Med. Chem. 15 (2007) 2396–2402. DOI:10.1016/j.bmc.2007.01.017 |
| [20] | M. Mizuno, T. Tanaka, N. Matsuura, M. Iinuma, C. Cheih, Two flavanones from Euchresta horsfieldii. Phytochemistry 29 (1990) 2738–2740. DOI:10.1016/0031-9422(90)85236-9 |
| [21] | P. Basnet, S. Kadota, S. Terashima, M. Shimizu, T. Namba, Two new 2-arylbenzofuran derivatives from hypoglycemic activity-bearing fractions of Morus insignis. Chem. Pharm. Bull. 41 (1993) 1238–1243. DOI:10.1248/cpb.41.1238 |
| [22] | M. Takasugi, S.I. Ishikawa, T. Masamune, Albafurans A and B, geranyl 2-phenylbenzofurans from mulberry. Chem. Lett. 11 (1982) 1221–1222. DOI:10.1246/cl.1982.1221 |
| [23] | Z.P. Zheng, S.B. Chen, S.Y. Wang, Chemical components and tyrosinase inhibitors from the twigs of Artocarpus heterophyllus. J. Agric. Food Chem. 57 (2009) 6649–6655. DOI:10.1021/jf9014685 |
| [24] | D. Lee, K.P.L. Bhat, H.H.S. Fong, Aromatase inhibitors from Broussonetia papyrifera. J. Nat. Prod. 64 (2001) 1286–1293. DOI:10.1021/np010288l |
| [25] | K. Shimizu, R. Kondo, K. Sakai, A stilbene derivative from Artocarpus incisus. Phytochemistry 45 (1997) 1297–1298. DOI:10.1016/S0031-9422(97)00143-X |
| [26] | S.H. Day, J.P. Wang, S.J. Won, C.N. Lin, Bioactive constituents of the roots of Cynanchum atratum. J. Nat. Prod. 64 (2001) 608–611. DOI:10.1021/np000428b |
| [27] | C.M. Li, Z.L. Liu, Q. Mu, Studies on chemical constituents from leaves of Goniothalamus griffithii. Acta Botan. Yunnan 19 (1997) 321–323. |
| [28] | J.W. Bok, L. Lermer, J. Chilton, H.G. Klingeman, G.H.N. Towers, Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 51 (1999) 891–898. DOI:10.1016/S0031-9422(99)00128-4 |
 2017, Vol. 28
2017, Vol. 28