b Department of Chemistry, Nanchang University, Nanchang 330031, China
Surface chemical reactions have been attracting wide-spread attention because these reactions bring new insights into traditional solvent chemical reactions and provide the possibility to synthesize molecules with novel properties. Surface-assisted reactions, such as metal-organic coordination [1-6], organometallic formation [7], Ullmann reactions [8-10], dehydrogenative homocoupling [11-13], Bergman cyclization [14], and cyclotrimerization of alkynes [15], have been investigated by various methods. Reactions involving carbon-carbon coupling are of particular interest as aryl groups are the principal components of aromatic molecules [16-19].
Great efforts have been undertaken to understand the reaction mechanisms of C-C coupling on single-crystal surfaces in ultrahigh vacuum (UHV) conditions. The observation of direct carbon-carbon coupling achieved via C-H activation has been reported in the case of alkane on Cu (110) [20] and quaterphenyl (4 ph) on Cu (110) [21]. However, other mechanisms of carbon-carbon coupling, such as in systems with both C-H and C-X (where X is a halogen atom) bonds have not been as extensively studied. Due to the inertness and poor selectivity of C-H bonds, halogen atoms are substituted to form C-X groups (X stands for halogen) as activation sites for Ullmann coupling, which has been exploited to build one-dimensional (1D) molecular wires [22-26], nanoribbons [27-29], or two-dimensional (2D) covalent organic networks [30-33].
In this letter, we report the direct observation of carbon-carbon coupling of 4, 4"-dibromo-p-terphenyl (DBTP) on Cu (110) surface at the single molecular level by scanning tunneling microscopy (STM). Our results demonstrate that the non-organometallic and organometallic intermediates coexist after annealing at 353 K. Further annealing to 393 K leads to the formation of two types of carbon-carbon couplings. The organometallic intermediates play a key role in Ullmann coupling. The direct carbon-carbon coupling arises from the selective C-H activation at meta sites of incompletely debrominated molecules.
2. ExperimentalThe experiments are carried out in a multi-chamber ultrahigh vacuum (UHV) system housing a SPECS variable temperature STM with a base pressure of less than 2 × 10-10 mbar. The Cu (110) surface is cleaned by several Ar+ sputtering (1.5 keV, 4.8-5.0 μA, 45 min) and annealing (900 K, 10 min) cycles. DBTP (Frontier Scientific, purity > 95%) held by Knudsen cells are degassed for hours before deposition. STM images are acquired at room temperature with a chemically etched W tip. Positive voltage indicates that the samples are biased positively with respect to the tip.
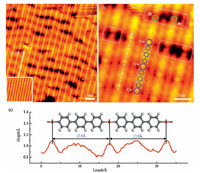
3. Results and discussion 3.1. The adsorption behaviors of DBTP on Cu (110)Fig. 1a shows a typical STM image of DBTP molecules adsorbed on Cu (110) after annealing to 353 K. The nestled parallel linear structure with some obvious cracks is observed along the [1-10] direction of the substrate. The high-resolution STM image (Fig. 1b) shows that the chains without cracks exhibit a periodic structure, in which the spindle-shaped features alternate with bright round features (denoted by blue cycles). From Fig. 1c, the height profile along the white dotted line in Fig. 1b, the distance between the two bright round dots is measured to be 15.9±0.5 Å, in agreement with the length of Cu-(ph)3-Cu (16.3 Å) from the structural model. The linear periodic structure corresponds to the organometallic intermediate in which (ph)3 units coordinate with Cu atoms and the longest intermediate comprises seven monomers. The fuzzy dot-like features embedded between these linear structures are identified as the Br atoms dissociated from the Br-(ph)3-Br molecules and adsorbed on the Cu (110) surface subsequently. It is worth noting that several dim round protrusions (denoted by green cycles) can be clearly visible at the cracks highlighted in the white dotted rectangles. Such dim protrusions are assigned as the Br atoms still attached to (ph)3 biradicals, indicating that the Br atoms within the DBTP molecules are not completely dissociated during the initial annealing process. These observations clearly demonstrate that the binding configuration of the DBTP molecules adsorbed on Cu (110) surface can be distinguished into non-organometallic and organometallic intermediates depending on whether these molecules are connected to each other via C-Cu-C bonds.
|
Download:
|
| Figure 1. (a) Large-scale, (b) high-resolution STM images of DBTP molecules on Cu (110) after annealing to 353 K. (c) The height profile along the white dotted line in (b). (scanning parameters: It=0.04 nA and Vt=-1400 mV). | |
3.2. Carbon-carbon coupling of DBTP on Cu (110)
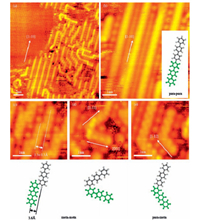
Further annealing at 393 K leads to the disappearance of the bright round dots within the organometallic intermediates and the formation of oligomers, as shown in Fig. 2a. The majority of the monomeric entities are linked together and present a smooth connection (density of states) in the middle part, indicating the covalent coupling nature between the molecules. A closer inspection of the STM image shows that three structural motifs are actually formed and denoted as para-para, meta-meta, and para-meta, respectively. The para-para motif in Fig. 2b is a linear structure along the [1-10] direction, implying that DBTP molecules are connected in a head-to-end way. This kind of oligomer with para-para motif represents the polymers synthesized by DBTP molecules via Ullmann reaction in which the carbon-carbon coupling occurs at the para sites of DBTP molecule after the release of the Cu atoms from the initial organometallic intermediates. As a consequence, the length of the poly (para-phenylene) oligomers (ph)3n is shorter than that of the linear organometallic intermediates containing the same number (n) of (ph)3 units. For the oligomers with the meta-meta motif, the molecules within this kind of oligomer are linked together in a shoulder-to-shoulder fashion, as shown in Fig. 2c and 2d. Under these configurations, the carbon-carbon coupling should take place at the meta-C sites of the terminal phenyl groups rather than the para-C sites. The intermolecular distance derived from the STM in Fig. 2c is 3.5±0.3 Åand in good agreement with the value (3.6 Å) from the structural model. Such selective coupling at meta-C sites has been found in the polymerization of 4 ph molecules on Cu (110) surface and the C-H activation at the meta-C site is suggested to be energetically most favorable [21]. We believe that the oligomers with the meta-meta motif in our study follow the similar reaction process. It is worth noting that the reaction of the same DBTP molecule on Cu (111) produces mostly para-para motifs [26], indicating that substrate lattice has an influence on the reaction products. In addition, the C-C coupling can also take place between the para-C site of the molecule along the [1-10] direction and the meta-C site of the molecule along the [1-12] direction with a small possibility. Consequently, the oligomers with para-meta motifs are formed on the surface and appear branch-shaped in Fig. 2e.
|
Download:
|
| Figure 2. (a) Large-scale STM image of DBTP molecules on Cu (110) after annealing to 393 K. (b-e) High-resolution STM images of frequently coupling motifs: (b) para-para motif, (c-d) meta-meta motif, (e) para-meta motif. (scanning parameters: It=0.04 nA and Vt=-1000 mV). | |
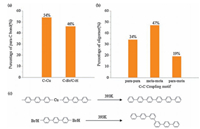
To understand whether the formation of the oligomers with various motifs is related to the initial configurations for the adsorbed molecules, statistical analysis has been done for the paraC bonding configuration of adsorbed molecules at 353 K and the C-C coupling configuration in the oligomers at 393 K. The statistical results on more than 2400 molecules demonstrate that the percentages of the para-C bonded to Cu atoms and to Br or H atoms are 46 and 54% (Fig. 3a), respectively. The statistics results on the final products in Fig. 3b give that the percentages of the oligomers with para-para, meta-meta, and para-meta motifs are 34, 47, and 19%, respectively. The para-C bonded to Cu atoms could be coupled together to form para-para motifs with the release of these Cu atoms via Ullmann reaction. In previous work [34], the para-C bonded to Cu atoms can also be coupled with the meta-C to form para-meta motifs. From the statistical results, the sum of the percentages of para-para (34%) and para-meta (19%) motifs is nearly equal to that of the para-C bonded to Cu atoms (54%), suggesting that these two reaction pathways are present in our study. On the other hand, the percentage of meta-meta motifs (47%) is close to the percentage of the para-C bonded to Br or H atoms (46%), indicating that the meta-meta motif originates from these para-C bonded to Br or H atoms. Therefore, we believe that the initial binding configuration plays a decisive role for the pathway of carbon-carbon coupling. As shown in Fig. 3c, the molecules within the organometallic intermediates tend to follow the Ullmann reaction to form the oligomers with para-para and para-meta motifs. For those molecules that do not participate in the formation of organometallic intermediates, the direct carbon-carbon coupling will be carried out at meta-C sites.
|
Download:
|
| Figure 3. (a) The percentages of the para-C bonded to Cu atoms and to Br or H atoms before complete coupling reaction. (b) The percentages of the oligomers with para-para, meta-meta, and para-meta motifs after complete coupling reaction. (c) The proposed coupling pathway of DBTP molecules on Cu (110). | |
4. Conclusion
In conclusion, we have investigated the carbon-carbon coupling of DBTP molecules on Cu (110) using scanning tunneling microscopy (STM). Our findings suggest that annealing at 353 K leads to the formation of a mixture of non-organometallic and organometallic intermediates of DBTP molecules. Further annealing to 393 K converts all the molecules into covalent oligomers with para-para, meta-meta, or para-meta motifs. The statistical results suggest that the initial binding configuration of the DBTP molecules decides the particular pathway of carbon-carbon coupling.
AcknowledgmentThis work was financially supported by Natural Science Foundation of China (Nos. 61474059, U1432129 and 11504158) and National Key Basic Research Program of China (No. 2013CB934200).
| [1] | T. Classen, G. Fratesi, G. Costantini, Templated growth of metal-organic coordination chains at surfaces. Angew. Chem. Int. Ed. 44 (2005) 6142–6145. DOI:10.1002/(ISSN)1521-3773 |
| [2] | D. Heim, K. Seufert, W. Auwä rter, Surface-assisted assembly of discrete porphyrin-based cyclic supramolecules. Nano Lett. 10 (2010) 122–128. DOI:10.1021/nl9029994 |
| [3] | H.H. Yang, Y.H. Chu, C.I. Lu, Digitized charge transfer magnitude determined by metal-organic coordination number. ACS Nano 7 (2013) 2814–2819. DOI:10.1021/nn4003715 |
| [4] | W.H. Wang, X.Q. Shi, S.Y. Wang, Cooperative modulation of electronic structures of aromatic molecules coupled to multiple metal contacts. Phys. Rev. Lett. 110 (2013) 046802.. DOI:10.1103/PhysRevLett.110.046802 |
| [5] | F.F. Xiang, C. Li, Z.P. Wang, Direct observation of copper-induced metalation of 5, 15-diphenylporphyrin on Au (111) by scanning tunneling microscopy. Surf. Sci. 633 (2015) 46–52. DOI:10.1016/j.susc.2014.11.021 |
| [6] | Z.L. Shi, N. Lin, Porphyrin-based two-dimensional coordination Kagome lattice self-assembled on a Au (111) surface. J. Am. Chem. Soc. 131 (2009) 5376–5377. DOI:10.1021/ja900499b |
| [7] | S. Haq, F. Hanke, M.S. Dyer, Clean coupling of unfunctionalized porphyrins at surfaces to give highly oriented organometallic oligomers. J. Am. Chem. Soc. 133 (2011) 12031–12039. DOI:10.1021/ja201389u |
| [8] | Q.T. Fan, C.C. Wang, Y. Han, Surface-assisted organic synthesis of hyperbenzene nanotroughs. Angew. Chem. Int. Ed. 52 (2013) 4668–4672. DOI:10.1002/anie.201300610 |
| [9] | L. Lafferentz, V. Eberhardt, C. Dri, Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 4 (2012) 215–220. DOI:10.1038/nchem.1242 |
| [10] | L. Grill, M. Dyer, L. Lafferentz, Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2 (2007) 687–691. DOI:10.1038/nnano.2007.346 |
| [11] | A.L. Pinardi, G. Otero, - Irurueta, I. Palacio, Tailored formation of N-doped nanoarchitectures by diffusion-controlled on-surface (cyclo) dehydrogenation of heteroaromatics. ACS Nano 7 (2013) 3676–3684. DOI:10.1021/nn400690e |
| [12] | M. Treier, C.A. Pignedoli, T. Laino, Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nat. Chem. 3 (2011) 61–67. DOI:10.1038/nchem.891 |
| [13] | A. Wiengarten, K. Seufert, W. Auw, ä rter, Surface-assisted dehydrogenative homocoupling of porphine molecules. J. Am. Chem. Soc. 136 (2014) 9346–9354. DOI:10.1021/ja501680n |
| [14] | Q. Sun, C. Zhang, Z.W. Li, On-surface formation of one-dimensional polyphenylene through Bergman cyclization. J. Am. Chem. Soc. 135 (2013) 8448–8451. DOI:10.1021/ja404039t |
| [15] | F.F. Xiang, Y. Lu, C. Li, Cyclotrimerization-induced chiral supramolecular structures of 4-ethynyltriphenylamine on Au (111) surface. Chem. Eur. J. 21 (2015) 12978–12983. DOI:10.1002/chem.201501434 |
| [16] | S.P. Chen, Y. Sun, S.B. Wan, T. Jiang, Facile synthesis of a 4-anilinoquinazoline dimer by Suzuki cross-coupling reaction. Chin. Chem. Lett. 22 (2011) 1033–1035. DOI:10.1016/j.cclet.2011.01.013 |
| [17] | C. Liu, C. Liu, X.M. Li, Z.M. Gao, Z.L. Jin, Oxygen-promoted Pd/C-catalyzed Suzuki-Miyaura reaction of potassium aryltrifluoroborates. Chin. Chem. Lett. 27 (2016) 631–634. DOI:10.1016/j.cclet.2015.12.022 |
| [18] | W.S. Zhang, W.J. Xu, F. Zhang, G.R. Qu, Synthesis of symmetrical 1, 3-diynes via tandem reaction of (Z)-arylvinyl bromides in the presence of DBU and CuI. Chin. Chem. Lett. 24 (2013) 407–410. DOI:10.1016/j.cclet.2013.03.016 |
| [19] | L. Zhou, Q.X. Xu, H.F. Jiang, Palladium-catalyzed homo-coupling of boronic acids with supported reagents in supercritical carbon dioxide. Chin. Chem. Lett. 18 (2007) 1043–1046. DOI:10.1016/j.cclet.2007.06.023 |
| [20] | Q. Sun, L.L. Cai, Y.Q. Ding, Dehydrogenative homocoupling of terminal alkenes on copper surfaces:a route to dienes. Angew. Chem. Int. Ed. 54 (2015) 4549–4552. DOI:10.1002/anie.201412307 |
| [21] | Q. Sun, C. Zhang, H.H. Kong, Q.G. Tan, W. Xu, On-surface aryl-aryl coupling via selective C-H activation. Chem. Commun. 50 (2014) 11825–11828. DOI:10.1039/C4CC05482B |
| [22] | C. Bombis, F. Ample, L. Lafferentz, Single molecular wires connecting metallic and insulating surface areas. Angew. Chem. Int. Ed. 48 (2009) 9966–9970. DOI:10.1002/anie.v48:52 |
| [23] | M. Di Giovannantonio, M. El Garah, J. Lipton-Duffin, Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined Ullmann polymerization. ACS Nano 7 (2013) 8190–8198. DOI:10.1021/nn4035684 |
| [24] | L. Lafferentz, F. Ample, H. Yu, Conductance of a single conjugated polymer as a continuous function of its length. Science 323 (2009) 1193–1197. DOI:10.1126/science.1168255 |
| [25] | J.A. Lipton-Duffin, O. Ivasenko, D.F. Perepichka, F. Rosei, Synthesis of polyphenylene molecular wires by surface-confined polymerization. Small 5 (2009) 592–597. DOI:10.1002/smll.v5:5 |
| [26] | W.H. Wang, X.Q. Shi, S.Y. Wang, M.A. Van Hove, N. Lin, Single-molecule resolution of an organometallic intermediate in a surface-supported Ullmann coupling reaction. J. Am. Chem. Soc. 133 (2011) 13264–13267. DOI:10.1021/ja204956b |
| [27] | A. Basagni, F. Sedona, C.A. Pignedoli, Molecules-oligomers-nanowiresgraphene nanoribbons:a bottom-up stepwise on-surface covalent synthesis preserving long-range order. J. Am. Chem. Soc. 137 (2015) 1802–1808. DOI:10.1021/ja510292b |
| [28] | J.M. Cai, P. Ruffieux, R. Jaafar, Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466 (2010) 470–473. DOI:10.1038/nature09211 |
| [29] | L. Talirz, H. Söde, J.M. Cai, Termini of bottom-up fabricated graphene nanoribbons. J. Am. Chem. Soc. 135 (2013) 2060–2063. DOI:10.1021/ja311099k |
| [30] | M. Bieri, M. Treier, J.M. Cai, Porous graphenes:two-dimensional polymer synthesis with atomic precision. Chem. Commun. (2009) 6919–6921. |
| [31] | M.O. Blunt, J.C. Russell, N.R. Champness, P.H. Beton, Templating molecular adsorption using a covalent organic framework. Chem. Commun. 46 (2010) 7157–7159. DOI:10.1039/c0cc01810d |
| [32] | R. Gutzler, H. Walch, G. Eder, Surface mediated synthesis of 2D covalent organic frameworks:1, 3, 5-tris (4-bromophenyl) benzene on graphite (001), Cu (111), and Ag (110). Chem. Commun. (2009) 4456–4458. |
| [33] | M. Bieri, M.T. Nguyen, O. Gröning, Two-dimensional polymer formation on surfaces:insight into the roles of precursor mobility and reactivity. J. Am. Chem. Soc. 132 (2010) 16669–16676. DOI:10.1021/ja107947z |
| [34] | K.J. Shi, D.W. Yuan, C.X. Wang, Ullmann reaction of aryl chlorides on various surfaces and the application in stepwise growth of 2D covalent organic frameworks. Org. Lett. 18 (2016) 1282–1285. DOI:10.1021/acs.orglett.6b00172 |
 2017, Vol. 28
2017, Vol. 28 


