The coordination chemistry of complexes of lanthanide nitrates with phosphine oxides has attracted interest for a number of years. The isolation of the complexes Ln (TRPO)3(NO3)3 and Ln (TRPO)2(NO3)3 (R represents alkyl groups, e.g. methyl, ethyl, butyl, octyl, cyclohexyl, isobutyl and isopropyl) shows that the 9-coordinate or 8-coordinate structures and the 3:1 or 2:1 molar ratios of ligand to metal ion are common in these systems [1-5]. For the lighter lanthanide (Ce, Pr, Nd, Eu) ions, 9-coordinate complexes Ln (TEtPO)3(NO3)3 were formed, while both 9-coordinate Ln (TEtPO)3(NO3)3 and 8-coordinate Ln (TEtPO)2(NO3)3 were formed for the heavier lanthanide (Tb and Ho) ions [6]. On an increase in the size of the ligand, 9-coordinate Ln (TRPO)3(NO3)3 (R=cyclohexyl [2] and isobutyl [3]) were formed throughout the lanthanide series. When the ligand was bulky TtBuPO (Tri-tertbutylphosphine oxide), 8-coordinate Ln (TtBuPO)2(NO3)3 instead of Ln (TtBuPO)3(NO3)3 were formed for all lanthanides [7]. Platt et al. [6, 8] found that solid state structures and solution properties depend on a balance between steric and electronic effects of TRPO and the size of the lanthanide ions.
Recently, several reviews, concerning the extraction of lanthanides in the ionic liquid (IL) based systems, were published [9-12]. As known, TRPO are traditional extractants with high extraction efficiency on lanthanides. In traditional solvent extraction, extensive studies on lanthanide complexes revealed that Ln (TRPO)3(NO3)3 and Ln (TRPO)2(NO3)3 are often formed [1, 5], but in the IL-based extraction systems, we have to take the influence of ILs on the extraction complexes into consideration. Now, the most widely used ILs are CnmimNTf2 (1-alkyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide) are the most widely used ILs. The influence of IL anion NTf2- on the structure of complexes is the focus study of this work [13-15].
Our research group has synthesized and characterized two Eu-containing ILs, i.e. [Eu (TBPO)4(NO3)2]NTf2 (TBPO: Tri-n-butylphosphine oxide) and [Eu (TOPO)4(NO3)2]NTf2 (TOPO: tri-n-octylphosphine oxide), and a single crystal [Eu (TPhPO)4(-NO3)2]NTf2 (TPhPO: triphenylphosphine oxide) [16]. However, the coordination structures of the above two Eu-containing ILs were just speculated from the single crystal structure of [Eu (TPhPO)4 (NO3)2]NTf2 characterized by X-ray diffraction measurements. On the basis of the above work, we tried to prepare the colorless crystal of X-ray quality, [Eu (TBPO)4(NO3)2]NTf2, by cooling the ethanol solution and recrystallization of the IL [Eu (TBPO)4 (NO3)2]NTf2. For comparison, we used the same method to get the single crystal [Gd (TBPO)4(NO3)2]NTf2. Although the complexes of lanthanide nitrates with tetra-TPhPO crystals have been studied [16, 17], to the best of our knowledge, there have been no reports so far on the tetra-TRPO crystals formed by Ln (NO3)3 with TRPO molecules. The two single crystals, therefore, are the focus on such 8-coordinate tetra-TRPO complexes.
2. Experimental 2.1. Materials and methodsAll chemicals were purchased commercially and used without further purification. The organic element analyses were performed on an elemental analyzer, vario EL (Elementar Analysensysteme GmbH, Germany). FT-IR spectra of the complexes were recorded on a NICOLET iN10 MX spectrometer. The thermogravimetric analyses (TGA) were measured on a Q600 SDT thermoanalyzer under N2 atmosphere with the temperature ranging from room temperature to 700 ℃ at a heating rate of 10 ℃ min-1. Powder X-ray diffraction (PXRD) data were measured on a DMAX-2400 diffractometer using Cu Kα radiation (λ=1.5406 Å) and the simulated data were carried out by the crystal analytic software ‘Mercury’. Solid-state fluorescence measurements were performed on an F-4500 (Hitachi) spectrophotometer. The crystallographic data for the single crystals were collected on an Agilent SuperNova Dual Atlas CCD diffractometer. Monochromated MoKα (λ=0.71073 Å) radiation was used. Using Olex2, the structure was solved with the XS structure solution program using direct methods.
2.2. Syntheses[Ln (TBPO)4(NO3)2]NTf2 (Ln=Eu, Gd) were formed during the reaction between Ln (NO3)3 (Ln=Eu, Gd) and ethanol solution containing both TBPO and LiNTf2 at 60 ℃. The products were colorless fluid at 30 ℃. Colorless crystals of X-ray quality were obtained upon recrystallisation from ethanol solution at 10 ℃. Anal. calcd. (found) for Eu (TBPO)4(NO3)2NTf2 (C50H108EuF6-N3O14P4S2): C, 42.01% (42.09%); H, 7.62% (7.46%); N, 2.94% (2.62%). Anal. calcd. (found) for Gd (TBPO)4(NO3)2NTf2 (C50H108EuF6-N3O14P4S2): C, 41.86% (41.99%); H, 7.59% (7.76%); N, 2.93% (2.89%).
3. Results and discussionThe molecular structures of [Ln (TBPO)4(NO3)2]NTf2 (Ln=Eu, Gd) are presented in Fig. 1. The two compounds have similar coordinate structures, in which the central metal ion is coordinated by four TBPO molecules and two bidentate nitrates. Four oxygen atoms of TBPO molecules are almost in a same plane, while two bidentate nitrates coordinate with the central metal ion oppositely. From the selected bond lengths data, the average distances of Eu-O (P) (Eu-O (P) band represents that the metal ion is coordinated with the oxygen atom of TBPO molecule) and Eu-O (N) (Eu-O (N) band represents that the metal ion is linked with the oxygen atom of the bidentate nitrate) bonds are 2.3194 Åand 2.9392 Å, respectively, while those of Gd-O (P) and Gd-O (N) are 2.3071 A ˚ and 2.9268 Å, separately. It is noted that NTf2- is not coordinated with the central metal ion, but functions as a counter anion. NTf2- can coordinate with lanthanides or alkaline earth metal ions through an oxygen atom of each sulfonyl group without the additional coordination of other molecules, and can also stabilize deficient transition metal or uranyl complexes via distinct modes [18]. A complex of Cs+ with a calixcrown bis (2-propyloxy) calix[4]crown-6 (BPC6) and NTf2-, in which NTf2- coordinates with Cs+ ion directly, has been synthesized and characterized in our previous work [19]. Our research group has been dedicating into the applications of ionic liquid on the extraction and separation of lanthanide ions. We have reported the effective extraction of Eu3+ by TRPO in CnmimNTf2 and the extraction species in the system was found to be [Eu (TBPO)4(NO3)2]NTf2 [16]. Lanthanide complexes Ln (TRPO)3(NO3)3 are often formed in traditional solvent extraction systems [1, 5]. In IL-extraction systems, however, the extraction species and extraction mechanism of TRPO are different from those of the normal organic solvent systems.

|
Download:
|
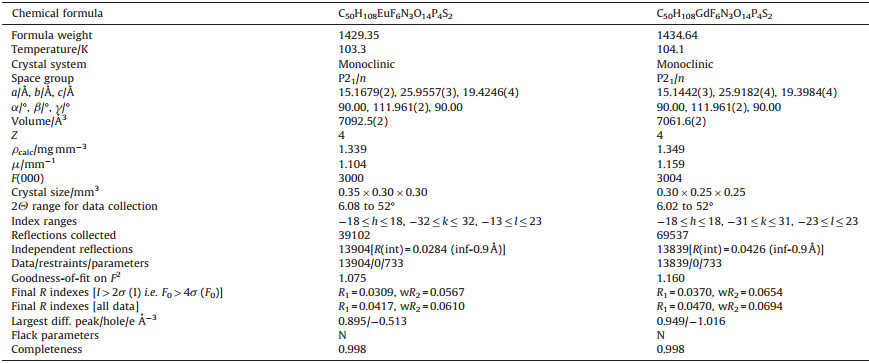
| Figure 1. Ellipsoid representation of the molecular structure of [Ln (TBPO)4(NO3)2]NTf2 (Ln=Eu, Gd). Selected bond lengths: Eu1-O1 2.3165(17); Eu1-O2 2.3516(17); Eu1-O3 2.3028(17); Eu1-O4 2.3065(17); Eu1-O5 2.5075(18); Eu1-O6 2.5075(18); Eu1-O8 2.5046(17); Eu1-O10 2.5557(17); Eu1-N1 2.925(2); Eu1-N2 2.953(2) Å. Gd1-O1 2.2973(19); Gd1-O2 2.302(2); Gd1-O3 2.3396(19); Gd1-O4 2.289(2); Gd1-O5 2.487(2); Gd1-O6 2.541(2); Gd1-O8 2.482(2); Gd1-O9 2.490(2); Gd1-N1 2.943(3); Gd1-N2 2.910(3) Å. | |
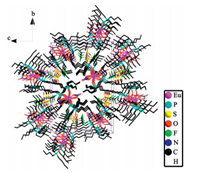
The crystal data and structure refinement of the two complexes are shown in Table 1. The two compounds are isostructural, with space group P21/n. In particular, the crystal cell parameters are basically same. In the crystal lattice (Fig. 2), the column like [Eu (TBPO)4(NO3)2]NTf2 molecules are arranged head-to-end along a direction, and further arrange in a nearly hexagonal closest packing extending along b and c directions. It is interesting to note that the crystal packing mode of [Gd (TBPO)4(NO3)2]NTf2 is consistent with that of europium complex. The two crystals were also investigated by powder X-ray diffraction (Fig. 3). The PXRD patterns of the two crystals are same. The main peaks of 2θ=6.368, 7.088, 8.068, 9.228, 10.148, 11.608, 13.048, 18.648 and 20.628 in the PXRD patterns agree well with the simulated patterns.
|
|
Table 1 Crystal data and structure refinement of [Ln (TBPO)4(NO3)2]NTf2 (Ln=Eu, Gd). |
|
Download:
|
| Figure 2. Packing of [Eu (TBPO)4(NO3)2]NTf2 molecules in the crystal structure, viewed along a direction. | |

|
Download:
|
| Figure 3. Experimental PXRD of single crystals [Eu (TBPO)4(NO3)2]NTf2 (1) and [Gd (TBPO)4(NO3)2]NTf2 (2), compared with the simulated results. | |
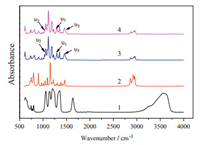
The infrared spectra (Fig. 4) of the two complexes contain some typical features of the phosphine oxide and nitrate. The two similar IR spectra results show the presence of bidentate nitrates (Scheme 1) in the complexes. The symmetric stretching mode (υ1) of the -NO2 group in the two complexes can be assigned at 1294 cm-1. Peaks of N-O stretching mode (υ2) appear at around 1030 cm-1. The asymmetric stretching mode (υ3) of the -NO2 group for the ionic liquid [Eu (TBPO)4(NO3)2]NTf2 is split (1466 and 1495 cm-1) [16], while the solid state [Eu (TBPO)4(NO3)2]NTf2 has no splitting at 1463 cm-1. The P-O stretch is observed at 1113 cm-1 for [Ln (TBPO)4(NO3)2]NTf2 (Ln=Eu, Gd), which is at lower wavenumbers than the corresponding P-O stretch of the free ligand TBPO (1154 cm-1). Other peaks wavenumbers like 1351 cm-1 and 1055 cm-1 are associated with the characteristics of NTf2- as compared with the IR spectra of LiNTf2.
|
Download:
|
| Figure 4. IR spectra of LiNTf2 (1), TBPO (2), [Eu (TBPO)4(NO3)2]NTf2 (3), [Gd (TBPO)4(NO3)2]NTf2 (4). | |
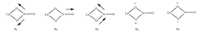
|
Download:
|
| Scheme 1. Stretching modes of NO3- in complexes. υ1, symmetric stretching mode of the -NO2 group; υ2, N-O stretching mode; υ3, asymmetric stretching mode of the -NO2 group; υ4 and υ5, non planar rocking frequency modes. | |
In order to assess the thermal stabilities and the phase behaviors of the two complexes, the decomposition temperatures were determined using TGA. The two complexes possess good thermal stability up to 250 ℃ due to the absence of phosphine oxides and nitrate ligands (Fig. 5). The TGA curves of [Eu (TBPO)4(-NO3)2]NTf2 and [Gd (TBPO)4(NO3)2]NTf2 show similar one-step weight loss profiles. In the temperature range 250-500 ℃, the two compounds rapidly decompose to their respective metal oxides. One-step weight loss is common in the TGA profiles of lanthanide compounds due to the continuous decomposition of constituents, such as Ln (TPhPO)2(phen)(NO3)3 (phen=1, 10-phenanthroline, Ln=Ce, Gd, Tb, Ho, Eu), but no optimal result of Eu (TPhPO)2 (phen)(NO3)3 could be obtained [20].

|
Download:
|
| Figure 5. TGA curves of [Eu (TBPO)4(NO3)2]NTf2 (1), [Gd (TBPO)4(NO3)2]NTf2 (2), TBPO (3), and LiNTf2 (4) (the curve of LiNTf2 cited from the Ref. [20]). | |
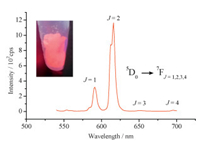
In addition, the crystal [Eu (TBPO)4(NO3)2]NTf2 exhibits excellent luminescent property as illustrated in Fig. 6. The strongest emission which splits into two peaks centered at 613 nm and 617 nm can be attributed to the forced electric dipole 5D0→7F2 transition of Eu3+ ions [21]. This is different from the sharp and narrow emission band at 616 nm of the emission spectrum of 1 mmol dm-3 [Eu (TBPO)4(NO3)2]NTf2 in acetonitrile [16]. The other three bands at 590, 651 and 696 nm correspond to the characteristic transitions for Eu3+ ion (5D0→7FJ, J=1, 3, 4) [22]. The emission spectrum of the crystal [Gd (TBPO)4(NO3)2]NTf2 on excitation at 243 nm shows one main band at 488 nm, corresponding to the 6F3/2→6P1/2 transition of Gd3+ ion (figure not shown). This emission spectrum is similar to the result reported in the literature [23].
|
Download:
|
| Figure 6. Emission spectrum of the crystal [Eu (TBPO)4(NO3)2]NTf2 at room temperature (λex=395 nm). Inset: the picture of the compound under UV radiation. | |
4. Conclusions
Two single crystals [Ln (TBPO)4(NO3)2]NTf2 (Ln=Eu, Gd) were synthesized and characterized. The infrared spectra of the compounds exhibited some typical features of the phosphine oxides and two bidentate nitrates. The TGA curves of two complexes showed similar one-step weight loss profiles. The two complexes exhibited good thermal stability up to 250 ℃, and then rapidly decomposed to their respective metal oxides in the temperature range 250-500 ℃. The crystal [Eu (TBPO)4(NO3)2]NTf2 showed excellent luminescent property and the strongest emission splitted into two peaks centered at 613 nm and 617 nm, attributing to the forced electric dipole 5D0→7F2 transition of Eu3+ ions. The two compounds have similar coordinate structures, in which the central metal ion is coordinated by four TBPO molecules and two bidentate nitrates, while NTf2- acts as the counter anion. The structural difference between [Ln (TBPO)4(NO3)2]NTf2 and Ln (TBPO)3(NO3)3 can help understand the new extraction mechanisms in the IL based systems as compared with the normal organic solvent systems.
AcknowledgmentThis work was supported by National Natural Science Foundation of China (No. 91226112). Dr. Zhixian Wang and Dr. Jian Hao are acknowledged for the element analyses and single-crystal Xray analyses measurements, respectively. The authors also thank Dr. Xueling Qiao and Haiwang Liu for their help in the crystal structure analyses and fruitful discussion.
| [1] | A.M.G. Massabni, M.L.R. Gibran, O.A. Serra, Phosphine oxides complexes of neodymium (Ⅲ) nitrate. Inorg. Nucl. Chem. Lett. 14 (1978) 419–427. DOI:10.1016/0020-1650(78)80008-7 |
| [2] | A.P. Hunter, A.M.J. Lees, A.W.G. Platt, Synthesis, structures and mass spectrometry of lanthanide nitrate complexes with tricyclohexylphosphine oxide. Polyhedron 26 (2007) 4865–4876. DOI:10.1016/j.poly.2007.06.023 |
| [3] | A. Bowden, P.N. Horton, A.W.G. Platt, Lanthanide nitrate complexes of tri-isobutylphosphine oxide:solid state and CD2Cl2 solution structures. Inorg. Chem. 50 (2011) 2553–2561. DOI:10.1021/ic102385p |
| [4] | A. Bowden, S.J. Coles, M.B. Pitak, A.W.G. Platt, Complexes of lanthanide nitrates with tri-isopropylphosphine oxide. Polyhedron 68 (2014) 258–264. DOI:10.1016/j.poly.2013.10.028 |
| [5] | V.K. Manchanda, K. Chander, N.P. Singh, G.M. Nair, Complexes of lanthanides with trioctylphosphine oxide and tributylphosphine oxide. J. Inorg. Nucl. Chem. 39 (1977) 1039–1041. DOI:10.1016/0022-1902(77)80260-1 |
| [6] | A. Bowden, K. Singh, A.W.G. Platt, Lanthanide nitrate complexes with triethylphosphine oxide. Solid state and solution properties. Polyhedron 42 (2012) 30–35. DOI:10.1016/j.poly.2012.04.021 |
| [7] | A. Bowden, S.J. Coles, M.B. Pitak, A.W.G. Platt, Complexes of lanthanide nitrates with tri tert butylphosphine oxide. Inorg. Chem. 51 (2012) 4379–4389. DOI:10.1021/ic300142r |
| [8] | A.W.G. Platt, K. Singh, The interactions between the sterically demanding trimesitylphosphine oxide and trimesityphosphine with scandium and selected lanthanide ions. J. Mol. Struct. 1111 (2016) 180–184. DOI:10.1016/j.molstruc.2016.01.073 |
| [9] | Y. Baba, F. Kubota, N. Kamiya, M. Goto, Recent advances in extraction and separation of rare-earth metals using ionic liquids. J. Chem. Eng. Jpn. 44 (2011) 679–685. DOI:10.1252/jcej.10we279 |
| [10] | X.Q. Sun, H.M. Luo, S. Dai, Ionic liquids-based extraction:a promising strategy for the advanced nuclear fuel cycle. Chem. Rev. 112 (2012) 2100–2128. DOI:10.1021/cr200193x |
| [11] | M.L. Dietz, Ionic liquids as extraction solvents:where do we stand?. Sep. Sci. Technol. 41 (2006) 2047–2063. DOI:10.1080/01496390600743144 |
| [12] | H.W. Liu, T. Yang, Q.D. Chen, X.H. Shen, Extraction behaviors of ionic liquid systems and application perspectives in reprocessing of spent nuclear fuel. J. Nucl. Radiochem. 37 (2015) 286–309. |
| [13] | M. Matsumiya, Y. Kikuchi, T. Yamada, S. Kawakami, Extraction of rare earth ions by tri-n-butylphosphate/phosphonium ionic liquids and the feasibility of recovery by direct electrodeposition. Sep. Purif. Technol. 130 (2014) 91–101. DOI:10.1016/j.seppur.2014.04.021 |
| [14] | G.L. Ma, W.J. Yuan, Z. Dong, Extraction of lanthanides from nitric acid solution using isobutyl-BTP/ionic liquid system. Nucl. Sci. Technol 26 (2015) S10305. |
| [15] | R. Turgis, G. Arrachart, V. Dubois, Performances and mechanistic investigations of a triphosphine trioxide/ionic liquid system for rare earth extraction. Dalton Trans. 45 (2016) 1259–1268. DOI:10.1039/C5DT03072B |
| [16] | H.W. Liu, Extraction Study of U and Eu in Ionic Liquid Systems and the Synthesis of Europium-Containing Ionic Liquid Complexes, Master Thesis, Peking University, Beijing, 2015. |
| [17] | W. Levason, E.H. Newman, M. Webster, Tetrakis (triphenylphosphine oxide) complexes of the lanthanide nitrates; synthesis, characterisation and crystal structures of. Polyhedron 19 (2000) 2697–2705. DOI:10.1016/S0277-5387(00)00588-X |
| [18] | T.X. Sun, Application of Ionic Liquids in the Extraction of Sr, Cs, U and Tc, Ph.D. Thesis, Peking University, Beijing, 2013. |
| [19] | T.X. Sun, Z.M. Wang, X.H. Shen, Crystallization of cesium complex containing bis (2-propyloxy) calix [4] crown-6 and bis[(trifluoromethyl) sulfonyl] imide, Inorg. Chim. Acta 390(2012) 8-11. |
| [20] | H.F. Li, B. Zheng, K.W. Huang, A new class of PN3-pincer ligands for metal-ligand cooperative catalysis. Coord. Chem. Rev. 293- 294 (2015) 116–138. |
| [21] | F.S. Chen, G. Chen, T. Liu, Controllable fabrication and optical properties of uniform gadolinium oxysulfate hollow spheres. Sci. Rep. 5 (2015) 17934. DOI:10.1038/srep17934 |
| [22] | J.H. Xue, X.H. Hua, L.M. Yang, Synthesis, crystal structures and luminescence properties of europium and terbium picolinamide complexes. Chin. Chem. Lett. 25 (2014) 887–891. DOI:10.1016/j.cclet.2014.01.012 |
| [23] | W.J. Sun, Y.X. Wu, H.G. Zhang, L. Zhu, S.L. Gao, Spectra of rare earth complexes with salicylate. Acta Photonica Sin. 35 (2006) 1593–1596. |
 2017, Vol. 28
2017, Vol. 28