The photopolymer science has witnessed the development and application in electronic or medical apparatus industry of photocuring technologies, such as optical fibers, coatings, adhesives, dental materials and tissue regeneration [1-6]. As one of the key components in the photo-curing systems, new photoinitiators [PIs] are still the subject of many researchers. Its rapid development is mainly driven by the advantages of economy and environment, such as rapid through cure, low energy requirements and solventfree formulations. Especially in many promising areas such as photoimaging, microelectronics, and holographic image recording and data storage, new photoinitiator systems with high initiation efficiency are highly demanded. Generally, photoinitiator systems can be divided into two types: (ⅰ) Type Ⅰ, known as a-cleavage, produce initiating radicals by bond cleavage upon radiation of light. (ⅱ) Type Ⅱ, undergo photo-excitation followed by an electron or hydrogen transfer process and consequently to form initiating species [7-9]. Two main approaches to develop new photoinitiator currently are the modification of existing photoinitiators and the design of new structures [10-14]. Yagci and Arsu’s groups have developed a series of new photoinitiators based on modification of thioxanthone or benzophenone [15-17]. Gescheidt et al. synthesized new structures based on benzaldoximes and acylgermanes as novel photointiator [18, 19]. Among the most studied photoinitiator systems are those based on PI/hydrogen or electron donor couples (Type Ⅱ). Recently, Laleve ´e group have developed naphthalimide derivatives/hydrogen donor/iodonium salt as hybrid photoinitiator for radical and cationic polymerization [20, 21].
Both Type Ⅰ and Type Ⅱ photoinitiators have their own characteristics. Usually, Type Ⅰ photoinitiator can produce free radical very quickly upon irradiation of UV-light with short wavelength. The UV-vis absorption of dyes as Type Ⅱ photoinitiator can be well tuned according to light sources such as highpressure Hg lamp and LED [22-24]. As the amount of radicals is the key to photopolymerization, to introduce photo-cleavable groups to Type Ⅱ photoinitiators might provide more radicals upon irradiation at the same time. So photoinitiation systems containing Type Ⅰ and Type Ⅱ groups will be efficient due to the synergistic effect.
Quinoxaline possesses a structure similar to a dye and can be classified as Type Ⅱ photoinitiators [25], and diphenylquinoxaline derivatives used as photoinitiators were investigated [26]. As our continuous work to develop high-efficient photoinitiator systems [27-31], in this text we explored quinoxaline containing photocleavable thioether group as hybrid photoinitiator. With the quinoxaline as a typical hydrogen abstraction group, we introduced phenyl thioether group into a series of quinoxaline skeletons, which was proved to be cleavable by irradiation of UV-light. This system possesses the advantage of both Type Ⅰ and Type Ⅱ mentioned above, such as the adjustable absorption wavelength and more active or not easily quenched radicals. The involved photochemical mechanisms were investigated by UV-vis photolysis, electron spin resonance spin trapping (ESR-ST) and high-performance liquid chromatography/mass spectrum (HPLC/ MS) techniques. Then we studied the kinetics for polymerization of multifunctional acrylates by Real Time Fourier Transform Infrared (FT-IR).
2. Experimental 2.1. Materials[1, 1'-Biphenyl]-3, 3', 4, 4'-tetraamine, 1, 2-bis (4-fluorophenyl) ethane-1, 2-dione, naphthalene-2, 3-diamine were obtained from TCI (Shanghai) Development Co., Ltd. Benzene-1, 2-diamine, [1, 1':2', 1"-terphenyl]-4', 5'-diamine were purchased from Meryer (Shanghai) Chemical Technology Co., Ltd. 2-Mercaptobenzothiazole (MBO) was obtained from J & K Chemical Co., (Shanghai, China). N-methyldiethanolamine (MDEA) and ethyl, 4-(dimethylamino)-benzoate (EDB) were purchased from Alfa Aesar. ((2-((2-(Acryloyloxy) propoxy) methyl) propane-1, 3-diyl) bis (oxy)) bis (propane-2, 1-diyl) diacrylate (G3POTA) and 2-phenoxyethyl acrylate (EM2102) were provided by Eternal Chemical Co., CTD. 2, 2-Bis (4-(acryloxypolyethoxy) phenyl) propane (A-BPE-10) was provided by Shin-Nakamura Chemical Co., Ltd. Other chemicals were obtained from China National Pharmaceutical Group Co. (Shanghai, China). All reagents were used as received except as noted.
2.2. Synthesis of 2, 3-diphenylquinoxaline derivativesFour phenylquinoxalines with thioether group (SQ), 2, 3-bis-(4-(phenylthio) phenyl) quinoxaline (SQ1), 2, 2', 3, 3'-tetrakis-(4-(phenylthio) phenyl) -6, 6'-biquinoxaline (SQ2), 2, 3-bis (4-(phenylthio) phenyl) benzo[g] quinoxaline (SQ3), and 2, 3-diphenyl-5, 6-bis (4-(phenylthio) phenyl) pyrazine (SQ4) weresynthesized according to Scheme 1, as well as phenyl quinoxalines (Q) as reference.Take SQ1 as example, 10 mmol of benzene-1, 2-diamine and 10 mmol 1, 2-bis (4-fluorophenyl)-ethane-1, 2-dioneweredissolved in 20 mL of ethanol in 85 ℃ and stirred for 12 h under nitrogen protection. The mixture was then evaporated to remove most of the solvent and recrystallized. The precipitant was filtered from ethanol and dried to get 2, 3-bis (4-fluorophenyl) quinoxaline (DF-Q).

|
Download:
|
| Scheme 1. Process for the synthesis of SQs based on quinoxalines and their structures. (ⅰ, 85 ℃ ethanol as solvent. ⅱ, DMF as solvent, toluene to take away moisture, KOH as catalyzer in 130 ℃). | |
10 mmol of DF-Q and three times equivalent of thiophenol (30 mmol) were put into 150 mL three-necked round bottom flask equipped with magnetic stirrer. After the addition of 50 mL N, N-dimethylformamide (DMF), one times equivalent of KOH and toluene were added to accelerate reaction rate. The reaction was under the gas protection and equipped with water separator to remove water. The mixture was stirred at 130 ℃ for 24 h. Appropriate addition of toluene should be done after the toluene was evaporated. The mixture was slowly trickled into 10 times ethanol. The precipitant was filtered from ethanol and dried to get 2, 3-bis (4-(phenylthio) phenyl) quinoxaline (SQ1).
2.3. Instruments1H NMR and 13C NMR spectra were recorded on a Varian Mercury Plus-400 nuclear magnetic resonance spectrometer (400 MHz) using CDCl3 and DMSO-d6 as solvents. FT-IR spectra were recorded on a Thermo Scientific IS10 Fourier Transform Infrared Spectrometer. Mass spectra were determined on ACQUITYTM UPLC & Q-TOF MS Premier Ultra Performance Liquid Chromatography & Quadrupole-Time-of-Flight Mass Spectrometer. UV-vis absorption spectra and dynamics were conducted on a Perkin-Elmer LS-50B spectrophotometer. SQs were dissolved in chloroform at a concentration about 5 × 10-5 mol/L. Elemental analysis was conducted on an Elementar Vario ELIII. ESR (electron spin resonance) experiments were carried out with a Bruker EMX EPR spectrometer.
2.4. ESR spin trapping (ESR-ST) experimentESR (electron spin resonance) experiments were carried out with a Bruker EMX EPR spectrometer at 9.5 GHz with a modulation frequency of 200 kHz with 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) as radical capturing agent. A high-pressure mercury lamp with a cut-off filter (365 nm) was used for irradiation in the ESR spectrometer cavity. The concentrations of SQ4 dissolved in dichloromethane were 1 × 10-4 mol/L. 25 μL of each sample was transformed into a quartz ESR tube and then purged with nitrogen to get rid of oxygen.
2.5. Radical photopolymerization with real time Fourier transform Infrared spectraPhotopolymerization of different monomers (Scheme 2) initiated by SQs was carried out while acquiring real time Fourier Transform Infrared Spectroscopy (FT-IR, Thermo Scientific IS10) with a high-pressure mercury lamp as the light source. The intensity of light is about 10 mW/cm2. Here, the sample consists of 4 mmol/L SQs and without any solvent. An approximately 2 mg film sample of 50 mm thickness was held on KBr for 3 min while irradiated under the protection of nitrogen. The change of Peak area in about 1640 cm-1 is directly proportional to the amount of acrylate reacted in the system. By integrating the area under the characteristic peak, the double bond conversion (DBC) of the HDDA was determined according to Eq. (1) [32, 33]:

|
Download:
|
| Scheme 2. Structure of coinitiators (MDEA, MBO, EDB) and multi-functional monomers (TMPTA, HDDA, EM2102, PPGDA-540, G3POTA, A-BPE-10). | |
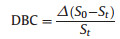
|
(1) |
Where St is the characteristic peak area in about 1640 cm-1 evolved at time t and S0 is the peak area before irradiation.
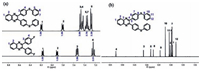
3. Results and discussion 3.1. Synthesis and photochemical properties of quinoxalinesFirstly, we synthesized phenyl thioether containing quinoxalines (SQs) according to Scheme 1. Fluorine substituted quinoxaline skeletons were synthesized through the condensation of benzil compounds and aromatic diamines compounds. Thioether group was formed through the reaction between phenyl fluorine and thiophenol. The resulting SQs were confirmed by NMR spectra (Fig. 1 and Fig. S1 in Supporting information), elemental analysis, ES/MS and FT-IR spectra (Fig. S2, Table S1 in Supporting information). Taking SQ1 as example, 1H NMR and 13C NMR spectra of SQ1 and its intermediate (DF-Q1) are shown in Fig. 1. As for 1H NMR of DF-Q1, the peak between 7.9 ppm to 8.2 ppm in the downfield can be assigned to the strong electron withdrawing effect of quinoxaline group. Two peaks at 7.25 ppm and 7.55 ppm can be assigned to phenyl fluorine. Compared with DF-Q1, the introduction of thioether group increased the electron density of phenyl ring. As shown in Fig. 1a, the appearance of three peaks between 7.2 ppm and 7.5 ppm corresponding to -C6H4-S indicates the successful introduction of thioether bond into quinoxaline group, which was also confirmed by the intensity ratio of signals. The structure of SQ1 was further determined by 13C NMR spectrum. The chemical shifts from 127 ppm to 153 ppm are well assigned to the labeled carbon atom in Fig. 1b and there are no excess miscellaneous peaks. The other three SQs were also confirmed as SQ1 (refer to Fig. S1). Purities higher than 90% for the five complexes were determined from these results.
|
Download:
|
| Figure 1. Partial NMR spectra of SQ1 and its intermediate product in DMSO-d6 (1H NMR, a) and CDCl3 (13C NMR, b). | |
The UV-vis spectra of the obtained SQs are presented in Fig. 2, and the detailed data of their photochemical properties are summarized in Table 1. Conjugate group plays an important rule in absorption wavelength. The maximal absorption wavelengths (λmax) of the four SQs are in the range of 350-450 nm. Due to the π-π* absorption of quinoxaline ring and large conjugate structure, their extinction coefficients (e) are all above 1 × 104. Along with the introduction of the conjugate groups, the phenomenon of red shift has occurred. For example, SQ2 has an obvious red shift compared with SQ1. As the conjugate group in SQ2 gives more space to let the π-electrons further away from the domain than SQ1, resulting in around 40 nm red-shift of λmax. Because the most commonly utilized irradiated wavelength of UV light is around 365 nm, the satisfactory UV absorption properties make SQs potential photoinitiators.

|
Download:
|
| Figure 2. UV-vis absorption spectra of five quinoxalines in CHCl3. | |
|
|
Table 1 UV-vis spectroscopic parameters of quinoxalines in chloroform |
We investigated the photo-cleavage behavior of SQs under irradiation of UV-light with Q as reference (Fig. 3, Fig. S3 in Supporting information). Indeed, upon irradiation by UV-light, SQs were cleavable, while Q kept almost unchanged. The absorption peak of SQs decreased with the increasing irradiation time. We supposed that the photo-cleavage behavior of SQs might be ascribed to the weak bond of C-S, which might leads to generation of free radicals. To confirm this point, we observed the DMPO/ radical adduct in an irradiated SQ4 or SQ4/EDB solution by ESR spin trapping (Fig. 4), which supports the generation of desired radicals through the SQ4 photo-cleavage and H-abstraction with EDB. In the presence of 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) as radical capturing agent, Fig. 4 shows that ESR signals had seven line spectrum, which is explained by a triplet with a-nitrogen and a further split into a doublet with a β-proton. The stronger intensity of ESR peak for SQ4/EDB suggests the higher amount of radicals generated after addition of EDB (Fig. 4b). The value of the magnetic parameter, factor-g, was 2.0081, thus indicating that the thioether or amine radicals had been released and trapped by DMPO [34].

|
Download:
|
| Figure 3. Photolysis experiments carried out in chloroform under the LED at 365 nm with gas protection. Evolution of the absorption spectrum as a function of time for SQ1 (a) and Q (b). | |

|
Download:
|
| Figure 4. ESR spin trapping spectra of the radicals generated in SQ4 (a) and SQ4/EDB (b) trapped by DMPO in CH2Cl2 upon the exposure to high-pressure mercury lamp with a cut-off filter (365 nm). Photolysis time is 5 min. | |
To further investigate its cleavage mechanism, we used HPLC/ MS to analyze SQ4 in acetonitrile before and after irradiation. As shown in Fig. 5, the total ion flow chart (TCI) spectra indicate the mass corresponding to quinoxaline structure before or after irradiation. Before irradiation, there is only one peak with a molecular weight of 602.1835 g/mol in HPLC-MS, which is corresponding to SQ4 (Fig. 5a). After irradiation, a peak with molecular weight of 386.1760 g/mol appears in HPLC-MS spectrum, which is corresponding to quinoxaline group (Fig. 5b). Comparing the two spectra and the different functional group weights in SQ4, it can be confirmed that the C-S bond close to quinoxaline group is photo-cleavable. The cleavage energy of thioether bond is~310 kJ/mol, which can be weakened by the electron withdrawing group. Therefore, the cleavage of thioether bond might be resulted from the energy transferred by the excited quinoxaline group [35-38].

|
Download:
|
| Figure 5. Total ion flow chart (TIC) of extracts of SQ4 in acetonitrile using High performance liquid chromatography mass spectrometry (HPLC-MS) before (a) and after UV irradiation at 365 nm for an hour (b). | |
3.2. Photopolymerization of monomers initiated by quinoxalines
Since SQs possess satisfactory characteristics as photoinitiator such as good absorption at UV-visible range with high molar extinction coefficient and the potential synergistic reaction of photo-cleavable and hydrogen-abstracting groups, we investigated photopolymerization of a series of multifunctional acrylate monomers initiated by SQs. To test the possibility of SQs as cleavable photoinitiators, we first studied the photopolymerization of HDDA initiated by SQs without hydrogen donor as coinitiator. As shown in Fig. 6, the real-time FT-IR profiles reveal that the photopolymerization behavior of HDDA appears to be similar to the other bifunctional monomers. The final conversion of HDDA for all four SQs is higher than 80%, indicating that SQs can be used as the efficient photointiator. On the contrast, photoinitiating efficiency of Q without thioether as reference photointiator is very low in the absence of coinitiator. These results further confirm that C-S bond of SQs can be broken to produce free radicals by irradiation of UV-light. It should be noted that the final conversion for SQ2 were more than 85%, while the polymerization induction period is longer than the other SQs (The rate of polymerization reaction relative parameters please refer to Table S2 in Supporting information). This might be ascribed to large conjugate structure with four cleavage thioether groups. The star-like structured SQ2 could provide more free radicals in the case of an equivalent to the other SQs, might leading to high conversion. On the other hand, the larger conjugate structure of SQ2 might lead to less solubility in HDDA, resulting in the less diffusion rate of free radicals and longer induction period.

|
Download:
|
| Figure 6. Photopolymerization profiles of HDDA in the presence of SQs under a 20 mL/min nitrogen flow, [SQs]=10 mmol/L. | |
The quinoxaline ring can abstract hydrogen from hydrogen donor to generate radicals due to its strong electron-accepting ability and excellent charge-transfer characteristics [39]. By initiating photopolymerization of HDDA in the presence of EDB hydrogen donor as coinitiator, we investigated photo-initiating efficiency of SQs as photoinitiator. Fig. 7 presents the photopolymerization profiles of HDDA initiated by four SQs as well as Q in. The final conversion of double bond for all four SQs is higher than 90%, indicating that SQ/EDB is very efficient photoinitiator system. Compared with SQs as photoinitiator, the higher efficiency of SQ/EDB might be ascribed to synergistic effect of photocleavable thioether groups and hydrogen-abstraction of quinoxaline groups, which can produce more free radicals under irradiation of UV-light. In the presence of EDB as hydrogen donor, the polymerization induction period for SQ2 become shorter, and the final conversion of double bond for SQ1, SQ3 and SQ4 is higher than 95%, much higher than that initiated by Q/EDB. Besides, photoinitiation system combining with EDB shows an obvious increase in the rate of polymerization (The rate of polymerization reaction and relative parameters please refer to Table S2). This indicated that the enhancement of initiating efficiency by EDB should be ascribed to the hydrogen-abstracting reaction between SQs and EDB, resulting in generation of more free radicals. Compared with Q/EDB reference, the much higher final conversion of double bond for SQs/EDB should be resulted from both photocleavage and hydrogen-abstraction.

|
Download:
|
| Figure 7. Photopolymerization profiles of HDDA in the presence of SQs/EDB under a 20 mL/min nitrogen flow. [SQs]=10 mmol/L, [EDB]=40 mmol/L. | |
Based on the photolysis and photopolymerization results reveled by UV-vis absorption, HPLC/MS and RT-FTIR spectra, the possible photoinitiation mechanism of SQ is proposed in Scheme 3. Upon irradiation of UV light, the excited SQs can cause cleavage of C-S bond to produce phenyl thiol radicals through Type Ⅰ approach, or abstract hydrogen from hydrogen donor through Type Ⅱ approach, resulting in formation of amino and quinoxaline radicals. Owing to the steric hindrance and delocalization of the unpaired electron, the quinoxaline radicals are usually not active toward vinyl monomers. The photopolymerization of vinyl monomers is usually initiated by phenyl thiol and amino radicals, which are rather active. Therefore, both thioether groups and hydrogen donor play an important role in SQ photoinitiator systems.

|
Download:
|
| Scheme 3. Proposed mechanism for photoinitiators based on quinoxaline. | |
To find the best hydrogen donor (coinitiator) for SQs, we investigated the photopolymerization of HDDA initiated by SQ1 in the presence of three coinitiators, EDB, MBO and MDEA. As shown in Fig. 8a, the final conversion of HDDA double bond for three SQ1 systems is higher than 90%, suggesting that EDB, MBO and MDEA are all efficient hydrogen donors for SQ1. Among these coinitiators, EDB is the most efficient one, while MBO is the preference in other detail [32]. Maybe aromatic secondary amine group have some interaction with the photolysis products and EDB radicals were more soluble in this system. Coinitiator content is also the key to photopolymerization. Less coinitiator could not achieve enough radicals, while excessive one may have some negative influences on curing product, such as molecule migration, increased cost and so on. Fig. 8b shows the real-time FT-IR profiles of photopolymerization of HDDA initiated by SQ1/EDB systems with different content of EDB. The final conversion of double bond was enhanced by the increasing content of EDB. The optimum proportion of SQ1 and EDB is 1:4 and increased content of EDB has no positive effect on photopolymerization.

|
Download:
|
| Figure 8. Photopolymerization profiles of HDDA in the presence of SQs/EDB, MBO, MDEA upon the high-pressure mercury lamp at 365 nm exposure under a 20 mL/min nitrogen flow. [SQ1]=10 mmol/L, [Co-i]=40 mmol/L (a). Photopolymerization profiles of HDDA in the presence of SQs/EDB upon the LED at 365 nm exposure under a 20 mL/min nitrogen flow. [SQ1]=10 mmol/L, [EDB]=20, 30, 40, 50 mmol/L (b). | |
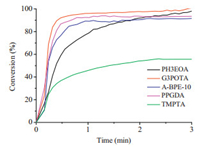
Motivated by the high photoinitiating efficiency of SQs in the presence of coinitiator, we studied the photopolymerization of various commercial acrylate monomers. Fig. 9 presents the realtime FT-IR profiles of photopolymerization of various commercial acrylate monomers EM2012, G3POTA, TMPTA, A-BPE-10 and PPGDA initiated by SQ1/EDB. The final conversion of double bond for all monomers is higher than 95%, except TMPTA with 55% conversion, indicating that SQs are very potential photoinitiators. As the UV-vis spectrum of SQ2 shows absorption peaks in the visible region, we investigated its initiation efficiency at the wavelength of 450 nm and the conversion of HDDA could be above 70% initiated by SQ2/EDB.
|
Download:
|
| Figure 9. Photopolymerization profiles of PH3EOA, G3POTA, A-BPE-10, PPGDA-540 and TMPTA in the presence of SQ1/EDB under a 20 mL/min nitrogen flow. [SQ1]=10 mmol/L, [EDB]=40 mmol/L. | |
4. Conclusion
We demonstrated that phenyl-thioether group containing diphenylquinoxaline derivatives (SQs) with photo-cleavable and hydrogen-abstracting groups in one molecule can be used as efficient hybrid photointiators. SQs possess suitable UV-vis absorption in the range of 350-400 nm with high extinction coefficients. The irradiation of UV-light can break the C-S bond in phenyl-thioether group of SQs. Photolysis and photopolymerization experiments revealed that SQs can be used as photo-cleavable photointiators, their photoinitiating efficiency can be enhanced by hydrogen donor. As photo-cleavable photoinitiators, SQs could initiate hexamethylene diacrylate (HDDA) very efficiently with the double bond conversion (DBC) of 80%. In the presence of ethyl-4-(dimethylamino) benzoate (EDB) as coinitiator, photoinitiator systems initiated photopolymerization of commercial acrylate monomers such as PH3EOA, G3POTA, HDDA, PPGDA, and A-BPE-10 with higher double bond conversion than 90%. It is believed that these characteristics provide SQs as potential photoinitiators in photo-curing field.
AcknowledgmentThe authors thank the National Basic Research Program (No. 2013CB834506) and National Nature Science Foundation of China (Nos. 51373098 and 21522403) for their financial support. X. Jiang thanks Mr. Kaji for discussions and the financial support from Hitachi-Chemical.
| [1] | C. Aydogan, M. Ciftci, Y. Yagci, Hyperbranched polymers by Type Ⅱ photoinitiated self-condensing vinyl polymerization. Macromol. Rapid Commun. 37 (2016) 650–654. DOI:10.1002/marc.v37.7 |
| [2] | X.H. Qin, A. Ovsianikov, J. Stampfl, R. Liska, Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMaterials 15 (2014) 49–70. |
| [3] | Y. Kou, J.Y. Wang, X.G. Jian, A novel epoxy methacrylate resin containing phthalazinone moiety for UV coatings. China Chem. Lett. 18 (2007) 598–600. DOI:10.1016/j.cclet.2007.03.024 |
| [4] | Y. Wang, P. Xiao, G.Q. Wu, S.Q. Shi, J. Nie, Photopolymerization induced by a benzophenone derivative photoinitiator. China Chem. Lett. 18 (2007) 977–980. DOI:10.1016/j.cclet.2007.06.012 |
| [5] | X.S. Jiang, J. Luo, J. Yin, A novel amphipathic polymeric thioxanthone photoinitiator. Polymer 50 (2009) 37–41. DOI:10.1016/j.polymer.2008.10.038 |
| [6] | F. Sun, Y.X. Li, N. Zhang, J. Nie, Initiating gradient photopolymerization and migration of a novel polymerizable polysiloxane ǁ-hydroxy alkylphenones photoinitiator. Polymer 55 (2014) 3656–3665. DOI:10.1016/j.polymer.2014.06.040 |
| [7] | R.X. Yin, K.M. Wang, J.W. Liu, J. Nie, 1, 3-dioxane methylcoumarin as a novel photoinitiator for free radical polymerization. J. Appl. Polym. Sci. 125 (2012) 2371–2375. DOI:10.1002/app.36491 |
| [8] | K.M. Wang, Y.H. Lu, P.H. Chen, J.S. Shi, H.N. Wang, Q. Yu, Novel one-component polymeric benzophenone photoinitiator containing poly (ethylene glycol) as hydrogen donor. Mater. Chem. Phys. 143 (2014) 1391–1395. DOI:10.1016/j.matchemphys.2013.11.051 |
| [9] | D.K. Balta, N. Arsu, Y. Yagci, Mechanism of photoinitiated free radical polymerization by thioxanthone-anthracene in the presence of air. Macromolecules 44 (2011) 2531–2535. DOI:10.1021/ma200147f |
| [10] | J. Lalev, F. Dumur, M.A. Tehfe, Dye photosensitized cationic ring-opening polymerization:search for new dye skeletons. Polymer 53 (2012) 4947–4954. DOI:10.1016/j.polymer.2012.08.067 |
| [11] | M.A. Tehfe, F. Dumur, B. Graff, Design of new Type Ⅰ and type Ⅱ photoinitiators possessing highly coupled pyrene-ketone moieties. Polym. Chem. 4 (2013) 2313–2324. DOI:10.1039/c3py21079k |
| [12] | S.J. Wang, X.D. Fan, Q.F. Si, J. Kong, G.B. Zhang, Synthesis and characterization of dendritic carbosilane based macrophotoinitiator. Acta Polym. Sin. 1 (2006) 707–711. |
| [13] | D.S. Esen, N. Arsu, J.P. Da Silva, S. Jockusch, N.J. Turro, Benzoin type photoinitiator for free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 51 (2013) 1865–1871. DOI:10.1002/pola.26569 |
| [14] | S. Kork, G. Yilmaz, Y. Yagci, Poly (vinyl alcohol)-thioxanthone as one-component type Ⅱ photoinitiator for free radical polymerization in organic and aqueous media. Macromol. Rapid Commun. 36 (2015) 923–928. DOI:10.1002/marc.v36.10 |
| [15] | S. Dadashi-Silab, C. Aydogan, Y. Yagci, Shining a light on an adaptable photoinitiator:advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 6 (2015) 6595–6615. DOI:10.1039/C5PY01004G |
| [16] | M. Aydin, G. Temel, D.K. Balta, N. Arsu, Mono" and "bifunctional" aromatic esterificated benzophenone photoinitiators for free radical polymerization. Polym. Bull. 72 (2015) 309–322. DOI:10.1007/s00289-014-1274-3 |
| [17] | B. Gacal, H. Akat, D.K. Balta, N. Arsu, Y. Yagci, Synthesis and characterization of polymeric thioxanthone photoinitatiors via double click reactions. Macromolecules 41 (2008) 2401–2405. DOI:10.1021/ma702502h |
| [18] | M. Griesser, A. Rosspeintner, C. Dworak, Initiators based on benzaldoximes:bimolecular and covalently bound systems. Macromolecules 45 (2012) 8646–8657. |
| [19] | D. Neshchadin, A. Rosspeintner, M. Griesser, Acylgermanes:photoinitiators and sources for Ge-centered radicals. Insights into their reactivity. J. Am. Chem. Soc. 135 (2013) 17314–17321. DOI:10.1021/ja404433u |
| [20] | J. Zhang, F. Dumur, P. Xiao, Structure design of naphthalimide derivatives:toward versatile photoinitiators for Near-UV/Visible LEDs, 3D printing, and water-soluble photoinitiating systems. Macromolecules 48 (2015) 2054–2063. DOI:10.1021/acs.macromol.5b00201 |
| [21] | J. Zhang, N. Zivic, F. Dumur, A benzophenone-naphthalimide derivative as versatile photoinitiator of polymerization under near UV and visible lights. J. Polym. Sci. Part A Polym. Chem. 53 (2015) 445–451. DOI:10.1002/pola.v53.3 |
| [22] | J. Zhang, M. Frigoli, F. Dumur, Design of novel photoinitiators for radical and cationic photopolymerizations under near UV and visible LEDs (385, 395, and 405 nm). Macromolecules 47 (2014) 2811–2819. DOI:10.1021/ma500612x |
| [23] | S.K. Do, ğ ruyol, Z. Do, ğ ruyol, N. Arsu, Thioxanthone based 9-[2-(methyl-phenylamino)-acetyl]-thia-naphthacene-12-one as a visible photoinitiator. J. Lumin. 138 (2013) 98–104. DOI:10.1016/j.jlumin.2013.01.037 |
| [24] | G. Temel, B. Aydogan, N. Arsu, Y. Yagci, Synthesis and characterization of onecomponent polymeric photoinitiator by simultaneous double click reactions and its use in photoinduced free radical polymerization. Macromolecules 42 (2009) 6098–6106. DOI:10.1021/ma901162y |
| [25] | N. Arsu, M. Ayd, Photoinduced free radical polymerization initiated with quinoxalines. Die Angew. Makromol. Chem. 270 (1999) 1–4. DOI:10.1002/(ISSN)1522-9505 |
| [26] | M. Ayd, N. Arsu, Photoinitiated free radical polymerization of methylmethacrylate by using of quinoxalines in the presence of aldehydes. Progr. Org. Coat. 56 (2006) 338–342. DOI:10.1016/j.porgcoat.2006.06.006 |
| [27] | X.S. Jiang, X.W. Luo, J. Yin, Polymeric photoinitiators containing in-chain benzophenone and coinitiators amine:effect of the structure of coinitiator amine on photopolymerization. J. Photochem. Photobiol. A:Chem. 174 (2005) 165–170. DOI:10.1016/j.jphotochem.2005.02.008 |
| [28] | X.S. Jiang, J. Yin, Water-soluble polymeric thioxanthone photoinitiator containing glucamine as coinitiator. Macromol. Chem. Phys. 209 (2008) 1593–1600. DOI:10.1002/macp.v209:15 |
| [29] | A.F. Luo, X.S. Jiang, J. Yin, Thioxanthone-containing renewable vegetable oil as photoinitiators. Polymer 53 (2012) 2183–2189. |
| [30] | H.H. Hou, Y.C. Gan, J. Yin, X.S. Jiang, Multifunctional POSS-based Nano-photoinitiator for overcoming the oxygen inhibition of photo-polymerization and for creating self-wrinkled patterns. Adv. Mater. Interfaces 1 (2014) 1400385. DOI:10.1002/admi.201400385 |
| [31] | L.D. Sun, X.S. Jiang, J. Yin, Study of methoxyphenylquinoxalines (MOPQs) as photoinitiators in the negative photo-resist. Progr. Org. Coat. 67 (2010) 225–232. DOI:10.1016/j.porgcoat.2009.12.005 |
| [32] | D.S. Esen, F. Karasu, N. Arsu, The investigation of photoinitiated polymerization of multifunctional acrylates with TX-BT by Photo-DSC and RT-FTIR. Progr. Org. Coat. 70 (2011) 102–107. DOI:10.1016/j.porgcoat.2010.10.010 |
| [33] | F. Karasu, M. Aydin, M.A. Kaya, D.K. Balta, N. Arsu, Determination of photoinitiated polymerization of multifunctional acrylates with acetic acid derivatives of thioxanthone by RT-FTIR. Progr. Org. Coat. 64 (2009) 1–4. DOI:10.1016/j.porgcoat.2008.07.004 |
| [34] | X.S. Jiang, H.J. Xu, J. Yin, Polymeric amine bearing side-chain thioxanthone as a novel photoinitiator for photopolymerization. Polymer 45 (2004) 133–140. DOI:10.1016/j.polymer.2003.10.058 |
| [35] | G. Martin, J. Ascanio, Gas-phase thermolysis of methyl t-butyl sulfide. React. Kinet. Catal. Lett. 43 (1991) 13–18. DOI:10.1007/BF02075405 |
| [36] | X. Zheng, E.M. Fisher, F.C. Gouldin, L. Zhu, J.W. Bozzelli, Experimental and computational study of diethyl sulfide pyrolysis and mechanism. Proc. Combust. Inst. 32 (2009) 469–476. DOI:10.1016/j.proci.2008.06.176 |
| [37] | G. Martin, N. Barroeta, Gas phase thermolysis of sulfur compounds. Ⅱ. Ditertiary butyl sulfide. Inter. J. Chem. Kinetics 12 (1908) 699–716. |
| [38] | S. Plaza, G. Celichowski, L. Margielewski, S. Leśniak, Flash thermolysis of dibenzyl and diphenyl disulphides. Wear 237 (2000) 295–299. DOI:10.1016/S0043-1648(99)00358-0 |
| [39] | D.K. Balta, S. Keskin, F. Karasu, N. Arsu, Quinoxaline derivatives as photoinitiators in UV-cured coatings. Progr. Org. Coat. 60 (2007) 207–210. DOI:10.1016/j.porgcoat.2007.07.024 |
 2017, Vol. 28
2017, Vol. 28 



