Titanium dioxide (TiO2) is an important semiconductor widely used in the fields of photocatalytic, optoelectronic and energy conversion due to its inherent superiorities, including environmental friendliness, low-cost and superior optoelectronic performance [1-10]. Considerable work has demonstrated that the structural and optical factors such as size, shape, band-gap and crystallinity of TiO2 could affect the performance of these applications [1, 11]. Therefore, different colloidal strategies have been developed to prepare a variety of micro- and nano-sized TiO2 crystals [12-18].To date, the non-aqueous approach has proven to be one of the most popular methods for precise and reproducible control of the size and shape of TiO2 nanocrystals [16, 17]. In these syntheses, different types of surfactants are often used to tailor the shape of TiO2 nanocrystals through the guidance of the growth along a specific direction. For example, Dinh et al. synthesized different-shaped TiO2nanocrystals by employing two different capping agents to control the growth rate of the different facets [17]. Furthermore, the shape and optical band gap of TiO2 nanocrystals can also be tuned by the use of fluoride due to the binding selectively to the {0 0 1} facet of anatase. For example, Yang et al.synthesized high-purity anatase crystals with a high percentage of reactive {0 0 1} facets by using TiF4 as the Ti sources and shape directing agent [19]. However, TiF4 is unstable making it difficult to manipulate/control. Therefore, it is necessary to explore substitutes to replace TiF4 as a fluorine source. To date, it has been rarely reported that different-shaped TiO2 nanocrystals are synthesized using fluoride, such as NaF and NH4F, as a shape directing agent. Herein, a simple solvothermal approach has been adopted to prepare anatase TiO2nanocrystals, in which oleic acid (OA) is used, not only capping agent, but also solvent. The shape of the TiO2 can be altered from nanorods to nano-ellipsoids by the addition of different amounts ofNaF, thus the optical band gap can also be changed due to the variation of fluorine content. However, when fluoride is changed to NH4F, the shape change is not evident as compared to that synthesized in the presence NaF due to the absence of fluorine on the surface of TiO2.
2. ExperimentalSynthesis of TiO2 nanocrystals: Different-shaped TiO2 nanocrystals were synthesized by using a solvothermal approach. Typically, 0.5 mmol of NaF was added to15 mL of olec acid (OA) in a three-necked flask, and then heated to 120 ℃ under magnetic stirring until the NaF was completely dissolved. Afterwards, the mixture was transferred into a 50 mL teflon-lined stainless steel autoclave, followed by quick injection of 1.5 mL of titanium butoxide (TBOT). The reaction mixture was heated at 180 ℃ and held for 18 h. Upon completion, the reaction was then cooled to r.t. naturally. The TiO2 nanocrystals were isolated by addition of absolute ethanol (Sigma-Aldrich) and centrifugation (8500 rpm/ 10 min). The washing and precipitation process was repeated twice by toluene and common ethanol, and then the resultant TiO2 nanocrystals were dried in vacuum at 60 ℃ overnight, or dispersed in toluene for further characterization. To study the effects of the amount of NaF on the shape of the resulting TiO2 nanocrystals, controlled experiments were performed using similar procedures, except the amount of NaF was changed to 0 and 0.2 mmol. Additionally, another controlled synthetic experiment using NH4F to replace NaF to determine the effect on TiO2 nanocrystals was initiated with the amount of NH4F kept at 0.5 mmol.

|
Download:
|
| Figure 1. XRD patterns ofTiO2 nanocrystals synthesized in the absence of fluoride and in presence of 0.5 mmol NaF and 0.5 mmol NH4F. The bottom vertical lines represent the standard diffraction peaks of anatase TiO2. | |
Sample characterization: The TEM images of the samples were collected by using a JEM-1400 transmission electron microscope operating at an accelerating voltage of 100 kV. The X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker D8 Discover X-ray diffractometer with a Cu Kα radiation source (λ=1.54056 Å). The XPS measurements were taken on a VG ESCALAB 220i-XL spectrometer with a 300 W Al Kα radiation source, and the binding energies for different elements were calibrated with respect to the C 1s line at 284.8 eV from the contaminant carbon. The absorption spectra of the products in toluene were obtained on a Varian Excalibur 3100 spectrophotometer. All the measurements were carried out under identical conditions at r.t.
3. Results and discussionFig. 1 shows the XRD patterns of the resultant TiO2 nanocrystals synthesized in the absence or presence of NaF or NH4F. All the diffraction peaks of the as-obtained products match well with the bulk TiO2 (JCPDS No. 21-1272), which can be indexed as a pure anatase phase with a tetragonal crystal structure. Broadening of the diffraction peaks reveals the nature of nanocrystals. The diffraction intensity of (0 0 4) peak is stronger than that of (2 0 0) peak, which indicates the anisotropy of the TiO2 nanocrystals [20]. The mean particle size can be calculated based on the Scherrer formula, the mean crystal size of the product synthesized in the presence of 0.5 mmol NaF can be calculated as 7.9 nm and 6.6 nm based on the (1 0 1) and (0 0 4) planes, respectively [10].
The TEM technique was employed to study the shape variation of the TiO2 nanocrystals, and Fig. 2 depicts the TEM images of TiO2 nanocrystals synthesized in the absence of NaF, in the presence of 0.2 and 0.5 mmol of NaF, and 0.5 mmol of NH4F. As shown in Fig. 2a, the resultant nanocrystals are nanorods with a mean length of 24.3 ± 3.4 nm and a diameter of 2.6 ± 0.5 nm, which indicates that the growth is along the {0 0 1} facet of the anatase phase, and is consistent with the previous report [20]. When 0.2 mmol of NaF was added into the reaction system, the product is mostly nanorods and their mean length is decreased to 11.7 ± 3.3 nm, but their mean diameter is increased to be 3.7 ± 0.7 nm. Additionally, some particles with ellipsoid-like shape are also observed. Further increasing the amount of NaF to 0.5 mmol in the reaction system leads to almost all of the product with an ellipsoid shape with the mean length further decreased to 9.3 ± 1.5 nm and the mean diameter increased to be 6.0 ± 0.9 nm, which is close to the XRD results based on the Scherrer formula. The aforementioned results suggest that the growth of {001}facets are restricted and the percentage of {1 01} facets in the TiO2 nanocrystals is increased with an increase in the amount of NaF added to the reaction system indicating the addition of fluoride plays an important role in the shape tenability of TiO2nanocrystals. To further confirm how fluoride affects the shape change of TiO2 in the non-aqueous reaction system, NH4F was also introduced to prepare TiO2 nanocrystals while other reaction conditions were unchanged. Fig. 2d depicts the TEM image of the resultant TiO2 nanocrystals synthesized in the presence of 0.5 mmol NH4F showing some nanorods and some small-sized nanocrystals coexist in the product. This result suggests that the effect of NH4F on the shape tenability of TiO2 nanocrystals is less than that of NaF and that the difference in the shape variation may arise from the different amount of HF released from the reaction. According to previous reports, HF was used as a shape directing agent, and the fluoride ion was often adsorbed selectively to the {0 0 1} facet of anatase TiO2, leading to the shape change of TiO2 [19]. With the introduction of NH4F to the reaction system, the amount of HF released is significantly reduced due to the increase of the pH value.

|
Download:
|
| Figure 2. TEM images of different-shaped TiO2 nanocrystals synthesized without and in the presence of fluoride: (a) without NaF; (b) 0.2 mmol NaF; (c) 0.5 mmol NaF and (d) 0.5 mmol NH4F. | |
The XPS technique was employed to study the quantification of the fluorine content and the chemical environment of fluorine atoms in the TiO2 nanocrystals synthesized without any NaF and in the presence of 0.5 mmol of NaF, and the corresponding XPS results of Ti 2p, O 1s and F 1s are given in Fig. 3a-c. Fig. 3a shows the two narrow and symmetrical peaks located at 464.4 and 458.7 eV for the two samples, which can be assigned to be the binding energies ofTi 2pi/2 and Ti 2p3/2, respectively. As shown in Fig. 3b, the O 1s peak can be fitted into two peaks located at 531.6 eV and 530.1 eV. The broad but weak peak at 531.6 eV can be attributed to Ti-OH species due to the hydrolysis process, and the narrow, but stronger peak at 530.1 eV, is associated with the O2- bound to Ti4+ [21, 22]. As shown in Fig. 3c, there is no F 1s signal detected in the product synthesized without NaF, but the F 1s peak at 684.5 eV is observed in the product synthesized in the presence of 0.5 mmol of NaF, which is consistent with fluorine bound to the surface of TiO2. The absence of the signal at 688.5 eV indicates that there is no F- in the lattice. According to XPS results, the atomic ratio of Ti to O can be estimated to be 1:1.97 and 1:1.94 for the TiO2 nanocrystals synthesized without any NaF and in the presence of 0.5 mmol of NaF, respectively.

|
Download:
|
| Figure 3. XPS spectra of TiO2 nanocrystals synthesized without NaF and in the presence of 0.5 mmol NaF: (a) Ti 2p; (b) O 1s and (c) F 1s. | |
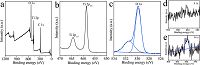
Fig. 4 depicts the XPS results of the TiO2 nanocrystals synthesized in the presence of 0.5 mmol of NH4F. As shown in Fig. 4d, the F signal is very weak which is hard to detect. Moreover, the O 1s peak is fitted into two peaks located at 531.4 eV and 529.8 eV (Fig. 4c), and the proportion of the oxygen related to Ti- OH is increased as compared to that of TiO2 nanocrystals synthesized in the presence of NaF, which can be attributed to the increase of pH value when the NH4F was introduced into the reaction system. The atomic ratio of Ti to O is estimated to be 1:2.14 derived from the XPS results of Ti 2p and O 1s, which indicates the increase of O content.
|
Download:
|
| Figure 4. XPS spectra of TiO2 nanocrystals synthesized in the presence of 0.5 mmol NH4F: (a) survey spectrum; (b) Ti 2p; (c) O 1s; (d) F 1s and (e) N 1s. | |
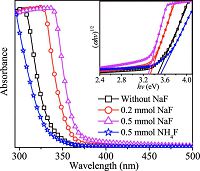
The optical band gap of the TiO2 nanocrystals can be estimated based on the absorption spectra, which are shown in Fig. 5. Compared with the absorption of TiO2 nanocrystals synthesized without any NaF, a noticeable red-shift of the absorption band is observed for the products synthesized in the presence of NaF, but the absorption shifts to shorter wavelength for the TiO2 nanocrystals synthesized in the presence of NH4F. As shown in the inset of Fig. 5, the TiO2 nanorods have an optical band gap of 3.47 eV, which is larger than bulk anatase TiO2 (3.2 eV), indicating the quantum confinement effect [20]. In contrast, the optical band gap of the ellipsoid-like TiO2 is estimated at 3.29 eV, which is smaller than TiO2 nanorods, which may arise from the fluorine adsorbed on the nanocrystal surface.
|
Download:
|
| Figure 5. Absorption spectra of TiO2 nanocrystals synthesized in the absence or presence of fluoride, and the inset shows the plot of band gap of each sample. | |
4. Conclusion
In summary, TiO2 nanorods and ellipsoid-like TiO2 nanocrystals have been synthesized by a modified solvothermal method, in which the introduction of NaF can release of HF binding selectively to the {0 0 1} facet of anatase TiO2. An increase of the amount of NaF leads to the shape variation from nanorods to ellipsoids, and the fluorine adsorbed on the surface of the products, but not doped into the lattices which can be confirmed by the XPS results. The introduction of NH4F has a little effect on the shape variation due to the little amount of HF released from the reaction system. The optical band gap of the TiO2nanocrystals is dependent on the shape and fluorine content.
Acknowledgments This work was partly supported by the Fundamental Research Fund of the Central Universities (Beijing JiaoTong University) (No. 2014JBZ010) and the author (AW) appreciates the support from the "Excellent One Hundred" project of Beijing JiaoTong University. The author (ZM) is also grateful to the National Undergraduates Training Programs for Innovation and Enterpreneuship.| [1] | Liu Y.D., Tang A.W., Zhang Q., Yin Y.D., Seed-mediated growth of anatase TiO2 nanocrystals with core-antenna structures for enhanced photocatalytic activity. J. Am. Chem. Soc. 137 (2015) 11327–11339. DOI:10.1021/jacs.5b04676 |
| [2] | Wang S.B., Pan L., Song J.J., Titanium-defected undoped anatase TiO2 with ptype conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 137 (2015) 2975–2983. DOI:10.1021/ja512047k |
| [3] | Dahl M., Liu Y.D., Yin Y.D., Composite titanium dioxide nanomaterials. Chem. Rev. 114 (2014) 9853–9889. DOI:10.1021/cr400634p |
| [4] | Liu H.Y., Joo J.B., Dahl M., Crystallinity control of TiO2 hollow shells through resin-protected calcination for enhanced photocatalytic activity. Energy Environ. Sci. 8 (2015) 286–296. DOI:10.1039/C4EE02618G |
| [5] | Wang H., Wang B.L., Ma S.Y., Synthesis of visible-light-driven TiO2 yolk-shell spheres with {001} facets dominated mesoporous shells. Chin. Chem. Lett. 24 (2013) 260–263. DOI:10.1016/j.cclet.2013.01.046 |
| [6] | Wang W.S., Sa Q.N., Chen J.H., Porous TiO2/C nanocomposite shells as a highperformance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 5 (2013) 6478–6483. DOI:10.1021/am402350n |
| [7] | Liu R., Wang P., Wang X.F., Yu H.G., Yu J.G., UV- and visible-light photocatalytic activity of simultaneously deposited and doped Ag/Ag(I)-TiO2 photocatalyst. J. Phys. Chem. C 116 (2012) 17721–17728. DOI:10.1021/jp305774n |
| [8] | Yu H.G., Chen W.Y., Wang X.F., Xu Y., Yu J.G., Enhanced photocatalytic activity and photoinduced stability of Ag-based photocatalysts:the synergistic action of amorphous-Ti(IV) and Fe(Ⅲ) cocatalysts. Appl. Catal. B:Environ. 187 (2016) 163–170. DOI:10.1016/j.apcatb.2016.01.011 |
| [9] | Wang X.F., Wang K., Feng K.W., Greatly enhanced photocatalytic activity of TiO2-xNx by a simple surface modification of Fe(Ⅲ) cocatalyst. J. Mol. Catal. A:Chem. 391 (2014) 92–98. DOI:10.1016/j.molcata.2014.04.015 |
| [10] | Xu Q.L., Yu J.G., Zhang J., Zhang J.F., Liu G., Cubic anatase TiO2 nanocrystals with enhanced photocatalytic CO2 reduction activity. Chem. Commun. 51 (2015) 7950–7953. |
| [11] | Joo J.B., Dahl M., Li N., Zaera F., Yin Y.D., Tailored synthesis of mesoporous TiO2 hollow nanostructures for catalytic applications. Energy Environ. Sci. 6 (2013) 2082–2092. DOI:10.1039/c3ee41155a |
| [12] | Yang S., Huang N., Jin Y.M., Crystal shape engineering of anatase TiO2 and its biomedical applications. CrystEngComm 17 (2015) 6617–6631. DOI:10.1039/C5CE00804B |
| [13] | Cargnello M., Gordon T.R., Murray C.B., Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem. Rev. 114 (2014) 9319–9345. DOI:10.1021/cr500170p |
| [14] | Cheng Z.L., Han S., Preparation of a novel composite electrode based on N-doped TiO2-coated NaY zeolite membrane and its photoelectrocatalytic performance. Chin. Chem. Lett. 27 (2016) 467–470. DOI:10.1016/j.cclet.2015.12.010 |
| [15] | Qian L., Jin Z.S., Yang S.Y., Du Z.L., Xu X.R., Bright visible photoluminescence from nanotube titania grown by soft chemical process. Chem. Mater. 17 (2005) 5334–5338. DOI:10.1021/cm050851b |
| [16] | Gordon T.R., Cargnello M., Paik T., Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 134 (2012) 6751–6761. DOI:10.1021/ja300823a |
| [17] | Dinh C.T., Nguyen T.D., Kleitz F., Do T.O., Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 3 (2009) 3737–3743. DOI:10.1021/nn900940p |
| [18] | Yan W.F., Chen B., Mahurin S.M., Dai S., Overbury S.H., Brookite-supported highly stable gold catalytic system for CO oxidation. Chem. Commun. (2004) 1918–1919. |
| [19] | Yang H.G., Sun C.H., Qiao S.Z., Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453 (2008) 638–641. DOI:10.1038/nature06964 |
| [20] | Joo J., Kwon S.G., Yu T., Large-scale synthesis of TiO2 nanorods via nonhydrolytic Sol-Gel ester elimination reaction and their application to photocatalytic inactivation of E. coli. J. Phys. Chem. B 109 (2005) 15297–15302. DOI:10.1021/jp052458z |
| [21] | Kalathil S., Khan M.M., Ansari S.A., Lee J., Cho M.H., Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 5 (2013) 6323–6326. DOI:10.1039/c3nr01280h |
| [22] | Chen X.B., Liu L., Yu P.Y., Mao S.S., Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331 (2011) 746–750. DOI:10.1126/science.1200448 |
 2016, Vol. 27
2016, Vol. 27 


