b Department of Pharmacology, Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing 100029, China ;
c Department of Pharmacy, China-Japan Friendship Hospital, Beijing 100029, China ;
d Classical Prescription Application Foundation Research Innovation Team, Beijing University of Chinese Medicine, Beijing 100029, China
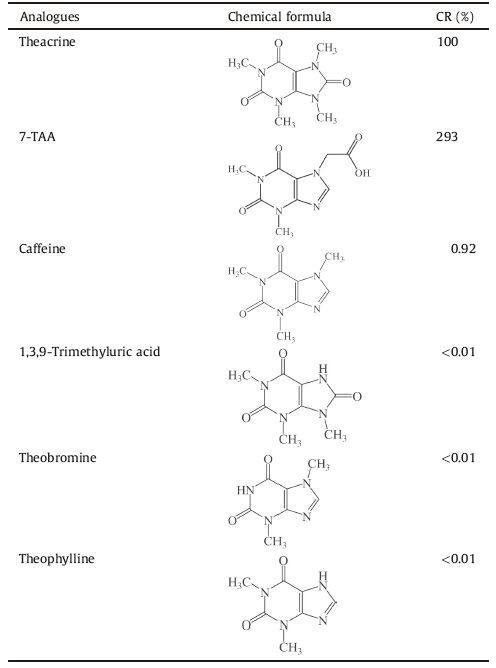
Tea is the most popular beverage in the world. The edible and medical function of tea has a long history in China. All types of tea including green tea, oolong tea, and black tea are manufactured from the same plant species, Camellia sinensis L. In the 1980s, Zhang found firstly that Camellia assamica var. kucha grows in wild woodlands at 1370 m altitude in Yaoshan village, Jinping country, Yunnan province of China [1]. Subsequently, further studies confirmed that these endemic tea plants contain larger quantities of theacrine (l, 3, 7, 9-tetramethyluric acid) [2]. Theacrine, a purine alkaloid similar to caffeine (Fig. 1), only exists in trace amounts in our daily drinking tea, Camellia sinensis and Camellia assamica. Initially, only rare trace theacrine were isolated from residues which came from several million pounds of tea [3, 4], and little attention was paid to it until the end of the last century.
|
Download:
|
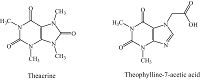
| Figure 1. Structures of theacrine and theophylline-7-acetic acid. | |
Purine alkaloids present in tea have significant effects on the central nervous system (CNS). Caffeine, theophylline, and theobromine are all widely used in clinical therapy. It is well known that caffeine and theobromine affect human psychomotor and cognitive performance [5, 6]. Studies of our lab and collaborators indicated that theacrine has potent hypnotic effects [7], anti-depressant effects [8], anti-inflammatory and analgesic effects [9], and a protective effect against stressprovoked liver damage [10]. In addition, further studies demonstrate that theacrine significantly enhances activity which is mediated by both the adenosinergic and dopaminergic systems [11].
To date, studies have addressed the quantitative analysis of theacrine by RP-HPLC [12] and HPLC-DAD/MS [13]. However, these methods need relatively large amounts of sample, complex pretreatment, and are time-consuming. Therefore, we developed an enzyme-linked immunosorbent assay (ELISA) method of theacrine quantification. Compared with HPLC analysis, ELISA of theacrine requires small sample amounts for detection (only about 10.0 mL blood at each time point) and can simultaneously analyze a large number of biological samples without pretreatment. ELISA based on monoclonal antibodies (MAbs) is suitable for quantitative analysis of theacrine in pharmacokinetic studies in small animals.
In this study, we developed an ELISA for determination of theacrine by using theophylline-7-acetic acid (7-TAA) (Fig. 1), a purine compound and structural analog of theacrine, to produce monoclonal antibodies. It has enough sensitivity and accuracy to detect a trace amount of theacrine in small quantities of biological material.
2. Experimental 2.1. ChemicalsTheacrine was extracted as previously described [14]. 7-TAA (purity 98%) was purchased from Nature Standard Ltd., Shanghai, China. Bovine serum albumin (BSA), ovalbumin (OVA), Freund's complete adjuvants, and Freund's incomplete adjuvants were purchased from Sigma-Aldrich, USA. Peroxidase-labeled antimouse immunoglobulin G (POD-IgG) was purchased from Gene- Script, Hong Kong, China. All other chemicals of analytical grade were standard commercial products.
2.2. Synthesis of antigen conjugates1-Ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC, 10 mg, 0.052 mmol), N-hydroxysuccinimide (NHS, 30 mg, 0.26 mmol), and 7-TAA (10 mg, 0.042 mmol) were added to 5.00 mL carbonate buffer and stirred for 12 hrs at room temperature. The reaction mixture was added drop wise to 1.0 mL carbonate buffer containing BSA (10 mg) and stirred for 12 hrs again at room temperature. Subsequently, the mixture was dialyzed against H2O with seven changes at room temperature. 7- TAA-OVA conjugates were also synthesized in the same manner, and lyophilized to get 8.25 mg 7-TAA-BSA and 8.96 mg 7-TAAOVA, respectively.
The 7-TAA-BSA was determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDITOF- MS), as previously described [14]. A small amount of an antigen conjugate was mixed with sinapinic acid in an aqueous solution containing 0.15% trifluoroacetic acid. The mixture was subjected to a Shimadzu Mass Spectrometer (Axima-CFR Plus) time-of-flight mass monitor, irradiated with a N2 laser at a linear mode mass spectrometer with laser energy 125.
2.3. Preparation of monoclonal antibodiesMale BALB/c mice (6 weeks old) were purchased from Vital River Laboratories, Beijing, China. Diet and water were provided ad libitum. All procedures and animal care practices were approved by the Committee on Ethics of Animal Experiments, School of Basic Medical Sciences, Beijing University of Chinese Medicine.
BALB/c male mice were immunized with the 7-TAA-BSA conjugates. The first immunization was administered into the back of BALB/c mice with 50 mg immunogen inH2Owith an equal volume of Freund's complete adjuvant. The second and third immunizations (50 mg protein per injection) were administered as a 1:1 emulsion in Freund's incomplete adjuvant bi-weekly. On the fourth day after the forth immunization (100 mg immunogen), the spleen was taken out to prepare splenocytes, and the splenocytes were fused with HAT-sensitive mouse myeloma cells, SP2/0, using the polyethylene glycol (PEG) method. Hybridomas producing MAbs reactive to theacrine were cloned using the limited dilution method. After cell culture, the cultured hybridoma was intraperitoneally injected into mice to produce ascites.
2.4. Indirect ELISA using anti-theacrine MAbs C11B5The reactivity of the MAbs to 7-TAA-OVA was determined by indirect ELISA. A 96-well microtiter plate (NUNC, Roskilde, Denmark) was coated with 100 mL of 1.0 mg mL-1 7-TAA-OVA conjugate in carbonate buffer (pH = 9.0) and incubated at 37 8C for 60 mins. The plate was washed three times with 0.05% Tween 20 containing PBS buffer (PBST). The plate was treated with 300 mL double-distilled water containing 10% gelatin for 1 h. Then, the plate was washed three times with PBST and reacted with 100 mL of testing antibodies for 1 h. Subsequently, the plate was washed again with PBST three times and combined with 100 mL of 104-fold diluted solution of peroxidase-labeled anti-mouse IgG for 0.5 h. After washing the plate six times with PBST, 100 mL of substrate solution, 0.1 mol mL-1 citrate buffer (pH 4.0) containing 0.003% H2O2, and 0.2 mg mL-1 3, 30, 5, 50-tetramethylbenzidine (TMB), were added to each well and incubated for 15 min. Absorbance at 450 nm was measured by a micro-plate reader.
2.5. Indirect competitive ELISA using anti-theacrine MAbs C11B5The indirect competitive ELISA procedure was conducted as follows: 7-TAA-OVA conjugate (100 mL, 1.00 mgmL-1 ) was adsorbed on the 96-well immunoplate, which was then treated with 300 mL of 10% gelatin. After being washed and blocked, 50 mL of the antibody and 50 mL of varying concentrations of either a standard analyte or the samples were added to each well. The plates were incubated, washed and measured with a micro-plate reader, as described above.
The cross-reactivities (CRs) of the MAbs against various compounds were evaluated and calculated as follows:
| ${\rm{CR}}\left( {\rm{\% }} \right){\rm{ = }}\frac{{{\rm{I}}{{\rm{C}}_{{\rm{50}}}}{\rm{for the acrine}}}}{{{\rm{I}}{{\rm{C}}_{{\rm{50}}}}{\rm{for compound under investigation}}}} \times {\rm{100\% }}$ |
where IC50 is the concentration of a test compound at A/A0 = 50%, with A and A0 being the absorbance values obtained in the presence and absence of the test compound, respectively.
2.6. Recovery of theacrineThe amount of theacrine in the unspiked sample was blank. Three concentrations of theacrine (8.75, 17.5, 25.0 mg mL-1) were spiked into blank samples in the same volume. The amount of theacrine in each sample was determined by the indirect competitive ELISA described above. The recovery was calculated from the measured amount and the added theacrine in the same concentration ranges as follows.
| ${\rm{Recovery}}\left( {\rm{\% }} \right)\frac{{{\rm{measured amount}}}}{{{\rm{added amount}}}} \times {\rm{100\% }}$ |
All animal studies were performed according to the Guidelines for the Care and Use of Laboratory Animals, and approved by the Committee of Ethics of Animal Experimentation of Beijing University of Chinese Medicine. 10 Kunming male mice weighting 25 ± 3.0 g were purchased from the Beijing Vital River Laboratory Animal Technology Limited Company (Beijing, China). Animals were housed in an environmentally controlled breeding room for 7 days with standard diet and water ad libitum. Mice fasted overnight before oral administration of a single dose of 45.0 mg kg-1 theacrine.
The sample (10 mL) which blooded from the mice tail vein, was dissolved in 190 mL sodium-carbonate buffer (pH 9.6). Then the experimental steps taken are as described in Section 2.4.
Pharmacokinetic parameters such as mean maximum blood concentrations (Cmax), time of maximum concentration (Tmax) and area under the curve (AUC0-t) were calculated by non-compartmental models using the DAS 2.0 software. All values were expressed as mean ± standard deviation (SD).
2.8. Comparison of HPLC and ELISA analyses.We conducted HPLC to compare its results to that of the developed ELISA analysis. To prepare the samples for HPLC we took the blood samples and 10 mL caffeine as the internal standard into 1.50 mL heparinized Ep tube, then added 1.00 mL ethyl acetate. The tube was vortex oscillated for 3 min, then centrifuged for 10 min for 1.34 × 10-4 r min-1 . 900mL of the supernatant was collected in another centrifuge tube, blow dried by N2, redissolved by 50% methanol compound solvent, vortex oscillated for 3 min, and finally centrifuged for 10 min for 1.34 × 10-4 r min-1 60 mL of the supernatant was used for HPLC detection.
An analytical HPLC system (Agilent 1100, DAD detector) was also used to confirm the theacrine concentrations in the blood samples under the following operating conditions: Agilent ZORBAX-C18 column (5 mm, 4.60 mm × 150 mm), column temperature was 25 8C. The mobile phase consisted of 23% CH3OH containing 0.01% formic acid. The flow rate was 1.00 mL min-1. The wavelength was 290 nm.
3. Results and discussion 3.1. Direct determination of 7-TAA-BSA conjugate by MALDI-TOF-MSAnalysis of 7-TAA-BSA conjugate by MALDI-TOF-MS is useful for precise determination of a hapten number of a conjugate. Fig. 2 shows peak with a center mass of 71.4 × 10-3 Da in the MALDI-TOF-MS spectrum. Given the fact that the molecular weight of BSA and 7-TAA-BSA were 66.4 × 10-3 and 71.4 × 10-3 Da, respectively, at least 21.0 molecules of 7-TAA were conjugated with each molecule of BSA. This average hapten number was within the range appropraite for immunisation.
3.2. Assay sensitivity and assay specificityEventually, one hybridoma secreting MAbs recognizing theacrinewas obtained, C11B5. The standard curve for theacrine was obtained by plotting A/A0 against the log of theacrine concentrations. Fig. 3 shows the full measuring range of the assay extended from 0.156-100 mg mL-1 . The regression equation was y = -0.160x + 0.933 with a correlation coefficient of 0.997. The half maximum inhibitory concentration (IC50) OD value was 1.55 mg mL-1.

|
Download:
|
| Figure 2. MALDI-TOF-MS spectrum of BSA and artificial antigen 7-TAA-BSA. | |

|
Download:
|
| Figure 3. Calibration curve of theacrine by ELISA. | |
Since cross-reactivity is the most important factor in determining the value of an antibody and dominates the specificity of an immunoassay, the cross-reactivities of the MAbs are examined by competitive ELISA. As indicated in Table 1, MAbs C11B5 had 100% cross-reactivity with theacrine, 293% cross-reactivity with 7-TAA, and 0.92% with caffeine. There was no detectable cross-reaction with other purine alkaloids commonly used in clinic. From these results, we conclude that this ELISA of MAbs can be widely useful for qualitative and quantitative analysis.
|
|
Table 1 Cross-reactivities of MAbs against six purine alkaloids commonly used in clinic. |
3.3. Assay variation
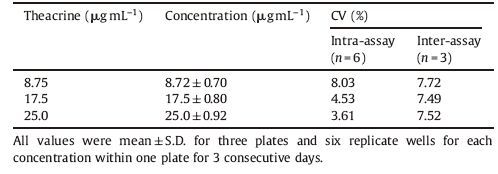
Reproducibility and precision are important criteria for an immunoassay. Standard curves for the competitive ELISA of theacrine from 3 consecutive days were compared, and the variations were calculated (Table 2). The variations between replicates from well to well (intra-assay) and plate to plate (interassay) were measured. Intra-assay precision was evaluated by the variation of determination of theacrine from well to well (n = 6) in the same plate and inter-assay precision was obtained from different plates (n = 3). From the result of Table 2, the maximum coefficient of variation (CV) intra-assay was 8.03%, while that of inter-assay was 7.72%.
|
|
Table 2 The accuracy and variation of assay. |
3.4. Recovery of theacrine from sample
Recovery experiments were performed to confirm the accuracy and reliability of the ELISA. Table 3 shows that theacrine recoveries in this experiment ranged from 99.7% to 100%. Each recovery of spiked theacrine from each sample was nearly 100%, which indicated a good accuracy of this ELISA method. This ELISA method was confirmed to be useful for reliable determination of theacrine in samples.
|
|
Table 3 The recovery rate of assay. |
3.5. Pharmacokinetic study
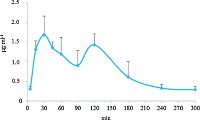
The ELISA method described above was applied to the analysis of theacrine in blood samples obtained from mice. The mean concentration-time profile in mouse blood after oral administration of theacrine at a single dose of 45.0 mg kg-1 is presented in Fig. 4. The pharmacokinetic characteristics were as follows: Area under the curve (AUC) 0-24 h, 271 ± 46.5 mg L-1 h; Cmax, 1.85 ± 0.40 mg L-1; Tmax, 0.750 ± 0.27 h. 3.6. Comparison of HPLC and ELISA analyses Table 4 shows that the two sets of data exhibited good correlation. This result further suggests the ELISA assay described here quantifies theacrine in small blood samples and it provides an alternative method for theacrine quantification.
|
Download:
|
| Figure 4. Mean concentration-time profile in mouse blood after oral administration of theacrine at a single dose of 45.0 mg kg-1 (mean ± SD, n = 6). | |
|
|
Table 4 Determination of theacrine by using ELISA and HPLC (n = 3). |
4. Conclusion
In summary, in this study we obtained specific monoclonal antibodies against theacrine, and successfully applied it to establish an ELISA. Compared with HPLC analysis, the benefits of ELISA of theacrine are rapid, simply, and cost-effective where a large number of samples can be determined without pretreatment. Moreover, the ELISA method was also successfully applied to the determination of theacrine concentration in the blood of mice.
Acknowledgment The research was financially supported by the following funds including the National Natural Science Foundation of China (Nos. 81473590, No. 81274115, No. 81473119), China-Japan Friendship Hospital Youth Science and Technology Excellence Project (No. 2014-QNYC-B-10), Program for New Century Excellent Talents in University of the Chinese Ministry of Education (No. NCET-11-0606), and Program for Excellent Talents of Beijing Municipal Party Committee Organization Department of the Communist Party of China (No. 2013D009999000001).| [1] | Zhang H.D., A revision on the tea resource plants. Acta Scientiarum Naturalium Universitatis Sunyatseni 23 (1984) 3–14. |
| [2] | Ye C.X., Lin Y.C., Su J.Y., Song X.H., Zhang H.D., Purine alkaloids in Camellia assamica var. kucha Chang et Wang. Acta Scientiarum Naturalium Universitatis Sunyatseni 38 (1999) 82–86. |
| [3] | Johnson T.B., Purines in the plant kingdom:the discovery of a new purine in tea. J. Am. Chem. Soc. 59 (1937) 1261–1264. DOI:10.1021/ja01286a030 |
| [4] | Petermann J.B., Baumann T.W., Metabolic relations between methylxanthines and methyluric acids in Coffea L. Plant Physiol. 73 (1983) 961–964. DOI:10.1104/pp.73.4.961 |
| [5] | Smith A., Brice C., Nash J., Rich N., Nutt D.J., Caffeine and central noradrenaline:effects on mood, cognitive performance, eye movements and cardiovascular function. J. Psychopharmacol. 17 (2003) 283–292. DOI:10.1177/02698811030173010 |
| [6] | Shi D., Daly J.W., Chronic effects of xanthines on levels of central receptors in mice. Cell. Mol. Neurobiol. 19 (1999) 719–732. DOI:10.1023/A:1006901005925 |
| [7] | Xu J.K., Kurihara H., Zhao L., Yao X.S., Theacrine, a special purine alkaloid with sedative and hypnotic properties from Cammelia assamica var. kucha in mice. J. Asian Nat. Prod. Res. 9 (2007) 665–672. DOI:10.1080/10286020601103155 |
| [8] | Xie G., Wu M.Z., Huang Y.R., Experimental study of theacrine on antidepressant effects. Chin. Pharmacol. Bull. 25 (2009) 1160–1163. |
| [9] | Wang Y.Y., Yang X.R., Zheng X.Q., Theacrine, a purine alkaloid with antiinflammatory and analgesic activities. Fitoterapia 81 (2010) 627–631. DOI:10.1016/j.fitote.2010.03.008 |
| [10] | Li W.X., Li Y.F., Zhai Y.J., Theacrine, a purine alkaloid obtained from Camellia assamica var. kucha, attenuates restraint stress-provoked liver damage in mice. J. Agric. Food Chem. 61 (2013) 6328–6335. DOI:10.1021/jf400982c |
| [11] | Feduccia A.A., Wang Y.Y., Simms J.A., Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine:involvement of adenosine and dopamine receptors. Pharmacol. Biochem. Behav. 102 (2012) 241–248. DOI:10.1016/j.pbb.2012.04.014 |
| [12] | Zhang W.K., Xu J.K., Hu J.Q., Determination of theacrine in rat plasma by RPHPLC. China J. Chin. Mat. Med. 38 (2013) 753–756. |
| [13] | Wang D.M., Du L.L., Lu J.L., HPLC-DAD/MS analysis of tea polyphenols in Camellia assamica var. kucha. Nat. Prod. Res. Dev. 18 (2006) 978–981. |
| [14] | Fukuda N., Tanaka H., Shoyama Y., Formation of monoclonal antibody against a major ginseng component, ginsenoside Rg1 and its characterization. Monoclonal antibody for a ginseng saponin. Cytotechnology 34 (2000) 197–204. DOI:10.1023/A:1008162703957 |
 2016, Vol. 27
2016, Vol. 27