The photochromic molecules have attracted increasing attention during the past decades because of their photo-reversibility [1-3]. Nondestructive readout is one of the most attractive aims [4] in optical materials for their applications in optic devices [5]. The fluorescent tuning has been a convenient mean of modulating the variable properties owing to the fast response times and high sensitivity [6, 7].
Photochromism is defined as reversible chemical transition of a molecule with irradiation upon appropriate light. Two isomers show different structures and absorption spectra. Many kinds of photochromic compounds have been reported in the past decades, including diarylethenes [8], spiropyrans [9], fulguides [10], stilbenes [11], azobenzenes [12], etc. Among above, the fulgides always exhibit favorable thermal stability, high fatigue resistance and reliable phohochromic reactivity in both solution and even solid state. The fulgides could be used to store binary information by ultraviolet and visible light for the application for photoinformation storage due to the excellent photochromic characteristics. However, two major problems should be resolved for the application while reading the stored data. Firstly, the exciting light of readout device would not be absorbed by either the open or the closed forms to avoid data damage in reading process. Secondly, the readout is based on the emissive luminescence which should not cause the photochemical reaction. Obviously, the direct method to resolve the dilemma is to separate the excitation and emission wavelengths from the absorption wavelengths of both isomers. In this article, the porphyrin was used as the luminescent center of fulgide and the method for nondestructive readout will be described in details.
Porphyrins [13] are known as natural molecules to exhibit characteristic Soret band in ultraviolet region and Qband in visible region and they will offer luminescence upon excitation. Therefore, they have been recognized as a class of popular luminescent materials that are applicable to bioassays, dyes, photosensitizer and other practical applications. In the case that porphyrin is used as the luminescent center in combination with a fulgide and there will be enough space with no overlap in energy and wave function between the two transitions. Hence, it would be apt to separate the excitation and luminescent fromthe two isomers. In this work, we will describe how a fulgide based on a porphyrin to constitute a photo-memory molecule with nondestructive readout capability. Porphyrins have been employed as fluorescent reporters in various photochromic molecular frames designed for the similar purposes [14-19].
2. ExperimentalTo a stirred solution of isopropylidene diethyl succinate (3.0 g, 14.0 mmol) in tetrahydrofuran (50 mL) at 0 ℃ under nitrogen was added NaH (0.8 g, 20.0 mmol) rapidly. The resulting mixture was stirred for 30 min. The solution of 5-chloro-2-methyl-3-acet- ylthiophene (1.74 g, 10.0 mmol) in THF (20 mL) was added and the mixture was allowed to warm to room temperature. After 8 h, the solvent was removed. The resulting brown syrup was dissolved in ethyl acetate (50 mL) and acidified with conc. HCl (10 mL). The reaction mixture was extracted with ethyl acetate (2 × 20 mL). The combined organic layers were dried (MgSO4) and filtered, and solvent was removed in vacuum. The semi-ester product was dissolved in 10% KOH-alcohol liquor (50 mL) and the solution refluxed for 2 h. Cold it to room temperature and acidified with conc. HCl (10 mL). The resulting mixture was extracted with ethyl acetate (2 × 20 mL). The combined organic layers were dried (MgSO4) and filtered, and solvent was removed in vacuum. The residue was dissolved in anhydrous dichloromethane and acet- ylchloride (2 mL) was added at ice-cold bath. After 8 h, the solvent was removed and afforded the target compound. Purification by chromatography on silica gel with petroleum ether: EtOAc (1:10) to yield fulgide 1.98 g (66.9%) as yellow solid. To a stirred solution of anhydrous CH2Cl2 of fulgide (30 mg, 0.1 mmol) and NH2-TPP (70 mg, 0.11 mmol), 1 mL of Et3N was added dropwise and the mixture was refluxed for 5 h. Cool it down to room temperature, and 2 mol/L HCl was added to the pH 4〜5. The resulting mixture was extracted with CH2Cl2 (2 × 20 mL) and washed with water (3 × 20 mL) to the pH 7. The combined organic layers were dried (MgSO4) and filtered, and solvent was removed in vacuum to yield 51 mg (59.2%) as amaranthine solid. The solid was dissolved in CH2Cl2 (15 mL) and 2 mL trifluoroacetic anhyride added. The solution turned red to green rapidly. The mixtures were stirred at room temperature for another 3 h. After reaction, the solution was removed in vacuum and the residue was washed with saturated NaHCO3 aqueous solution, extracted with CH2Cl2, dried with MgSO4 and filtered. The solution was removed in vacuum. Purification by chromatography on silica gel with petroleum ether: CH2Cl2 (5: 1) to yield 48 mg (51.6%) as violet solid. mp: > 250 ℃.1H NMR (CDCl3, 500 MHz): d 9.04 (s, 2H), 8.78 (s, 6H), 8.41 (s, 2H), 7.92〜7.89 (m, 17H), 7.17 (s, 1H), 2.85 (s, 3H), 2.52 (s, 3H), 2.23 (s, 3H), 1.21 (s, 3H), -2.60 (s, 2H); 13C NMR (CDCl3, 127 MHz): d 167.6, 166.6, 166.1, 159.5, 146.8, 142.3, 137.0, 135.6, 134.4, 133.7, 132.0, 131.9, 131.0, 129.1, 128.6, 128.1, 127.9, 125.9, 124.3, 120.1, 119.7, 22.0, 21.8, 14.6; IR(KBr) n: 3455, 3369, 3315, 3052, 3023, 2918, 1812, 1616, 1596, 1510, 1470, 1440, 1349, 1279, 1176, 979, 796, 729, 698 cm-1; MS(ESI, m/z):908.24 [M + H]+.
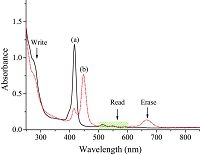
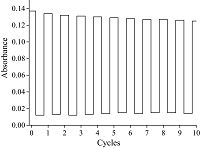
3. Results and discussionFor the first time, the structure and isomerization of the FUL- TPP used in this work are shown in Scheme 1. The open form of FUL-TPP in CH2Cl2 has nearly no absorption band in the visible region. Upon irradiation with UV light (λ=254 nm), the pink solution turned kelly and a new absorption band appeared around 667 nm ranging from 637 nm to 714 nm. Another interesting phenomenon shown in Fig. 1 is that the absorption in Soret band of porphyrin red shift from 418 nm to 448 nm of FUL-TPP due to the increasing electron density from the gradual formation of the closed isomer. The green solution was bleached in a large extent after irradiation with visible light, and a small protuberance arises at nearly 273 nm. These ring closing and opening cycles were repeated at least 10 times (Fig. 2). Therefore, the FUL-TPP showed feasible reversible photochromic reaction (Scheme 2).
|
Download:
|
| Figure 1. UV–Vis absorption spectra of a CH2Cl2 solution of the FUL-TPP: (a) open form, (b) closed form. | |
|
Download:
|
| Figure 2. Photocycling of the FUL-TPP in solution of CH2Cl2. The absorbance at l = 667 nm measured for 5 s after each switching operation are shown: after UV irradiation for 2 min (high value) and after visible light irradiation for 20 min (low value). | |

|
Download:
|
| Scheme. 1. Photochemical transition of isomeric forms of the FUL-TPP. | |

|
Download:
|
| Scheme. 2. Synthetic route of the FUL-TPP. | |
The compound FUL-TPP-O can emit fluorescence at 627 nm when induced with the UV-vis region (Fig. 3). The excitation wavelengths exist in a window from 500 to 600 nm. Accordingly, their closed isomeric forms luminescence quenched while excited at the corresponding light. Meanwhile, both FUL-TPP-O and FUL- TPP-C would not be converted to each other under irradiation with the light in this region. Hence, a nondestructive readout method would be achieved. Moreover, the emission intensity could be modulated by actinic reaction between the open and closed isomers.

|
Download:
|
| Figure 3. Emission spectra of FUL-TPP in solutions of CH2Cl2 (a) the open form and (b) the closed form. λex = 530 nm, λem = 627 nm. | |
Furthermore, the solution of two isomers of FUL-TPP were exposed under the light (λ=530 nm) for 0.5 h. The distinction of UV-vis absorption and luminescence spectra from that before irradiation were not observed. These results indicated that emission of porphyrins prefer to transfer the energy to the π-conjugated groups without damage on the primary construction rather than energy communication between adjacent porphyrins.
In a general way, the solutions of the open form of great majority of fulgides are colorless or yellow, and the closed forms are usually dark colored. In this work, the open form of the solution of CH2Cl2 of FUL-TPP is light red that is because the porphyrinic derivatives are generally close to red. However, the color of the closed-ring form is green that is different from our prediction. In order to investigate the reason of the distinction, we designed the procedures as follows. Firstly, we prepared a series of solutions of CH2Cl2, THF, EtOAc and DMSO of FUL-TPP in about 10~7mol/L. They are all nearly colorless but with a little bit red. After irradiation with light at 254 nm for 20 min, they all turned dark red except the solution of CH2Cl2. We predict that the reason may be the generation of acid by the irradiation upon UV light on CH2Cl2. In order to confirm our prediction, double solutions of CH2Cl2 was prepared and one of them was irradiated upon the UV light followed the addition of K2CO3 powder. To the other one, equal quantity of acetic acid was added carefully and the interface of the two solutions turned green. After vibration, the solutions blend to one phase and show green, then were moved to the UV light. After irradiation for 20 min, the first one with K2CO3 turned dark red and the second one with CH3COOH turned dark green. To the first one, the equal quality of glacial acetic acid was added, after vibration, the solution turned green. The second solution turned red after addition of ammonium hydroxide. The result indicates that the solution of CH2Cl2 of FUL-TPP shows red in acid and green in basic condition. The irradiation of UV light makes it turned from light to dark and blenched in visible light (Fig. 4).

|
Download:
|
| Figure 4. The color changes of FUL-TPP in solutions CH2Cl2. | |
4. Conclusion
In summary, this work has shown that each of the two isomers of FUL-TPP exhibit the distinctions on structures and spectra. The nondestructive readout method is clearly demonstrated when appropriate light is handled followed by detection of the responding emission after writing binary data for the application in optical information storage and any more photoelectricity- control devices.
| [1] | Irie M., Diarylethenes for memories and switches. Chem. Rev. 100 (2000) 1685–1716. DOI:10.1021/cr980069d |
| [2] | Matsuda K., Irie M., Diarylethene as a photoswitching unit. J. Photoch. Photobio. C 5 (2004) 169–182. DOI:10.1016/S1389-5567(04)00023-1 |
| [3] | Tian H., Wang S., Photochromic bisthienylethene as multi-function switches. Chem. Commun. 38 (2007) 781–792. |
| [4] | Pascal V., Justine W., Sophie B., Thomas B., Versatile and nondestructive photochemical process for biomolecule immobilization. Langmuir 29 (2013) 2075–2082. DOI:10.1021/la304941a |
| [5] | Sanghoon K., Seong-Jun Y., Soo P., Young, Highly fluorescent chameleon nanoparticles and polymer films:multicomponent organic systems that combine FRET and photochromic switching. J. Am. Chem. Soc. 134 (2012) 12091–12097. DOI:10.1021/ja3027295 |
| [6] | De-Silva A.P., Gunaratne H.Q.N., Gunnlaugsson T., Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97 (1997) 1515–1566. DOI:10.1021/cr960386p |
| [7] | Anthony S.P., Organic solid-state fluorescence:strategies for generating switchable and tunable fluorescent materials. ChemPlusChem 77 (2012) 518–531. DOI:10.1002/cplu.v77.7 |
| [8] | Chan J.C., Lam W.H., Wong H.L., Diarylethene-containing cyclometalated platinum(Ⅱ) complexes:tunable photochromism via metal coordination and rational ligand design. J. Am. Chem. Soc. 133 (2013) 12690–12705. |
| [9] | Buback J., Kullmann M., Langhojer F., Ultrafast bidirectional photoswitching of a spiropyran. J. Am. Chem. Soc. 132 (2010) 16510–16519. DOI:10.1021/ja1062746 |
| [10] | Tomasello G., Bearpark M.J., Robb M.A., Orlandi G., Garavelli M., Significance of a zwitterionic state for fulgide photochromism:implications for the design of mimics. Angew. Chem. Int. Ed. 49 (2010) 2975–2978. DOI:10.1002/anie.200906978 |
| [11] | Stoll R.S., Peters M.V., Kuhn A., Photoswitchable catalysts:correlating structure and conformational dynamics with reactivity by a combined experimental and computational approach. J. Am. Chem. Soc. 131 (2009) 357–367. DOI:10.1021/ja807694s |
| [12] | Sebai S.C., Milioni D., Walrant A., Photocontrol of the translocation of molecules, peptides, and quantum dots through cell and lipid membranes doped with azobenzene copolymers. Angew. Chem. Int. Ed. 51 (2012) 2174–2178. |
| [13] | Seol M.L., Choi S.J., Choi J.M., Ahn J.H., Choi Y.K., Hybrid porphyrin-silicon nanowire field-effect transistor by opto-electrical excitation. ACS Nano 6 (2012) 7885–7892. DOI:10.1021/nn303260a |
| [14] | Norsten T.B., Branda N.R., Axially coordinated porphyrinic photochromes for nondestructive information processing. Adv. Mater. 13 (2001) 347–349. DOI:10.1002/(ISSN)1521-4095 |
| [15] | Myles A.J., Branda N.R., Controlling photoinduced electron transfer within a hydrogen-bonded porphyrin-phenoxynaphthacenequinone photochromic system. J. Am. Chem. Soc. 123 (2001) 177–178. DOI:10.1021/ja002733p |
| [16] | Tsuchiya S., Intramolecular electron transfer of diporphyrins comprised of electron-deficient porphyrin and electron-rich porphyrin with photocontrolled isomerization. J. Am. Chem. Soc. 121 (1999) 48–53. DOI:10.1021/ja980677a |
| [17] | Terazono Y., Kodis G., André asson J., Photonic control of photoinduced electron transfer via switching of redox potentials in a photochromic moiety. J. Phys. Chem. B 108 (2004) 1812–1814. DOI:10.1021/jp037005d |
| [18] | Straight S.D., André asson J., Kodis S.G., Molecular AND and INHIBIT gates based on control of porphyrin fluorescence by photochromes. J. Am. Chem. Soc. 127 (2005) 9403–9409. DOI:10.1021/ja051218u |
| [19] | Kärnbratt J., Hammarson M., Li S., Photochromic supramolecular memory with nondestructive readout. Angew. Chem. Int. Ed. 49 (2010) 1854–1857. DOI:10.1002/anie.v49:10 |
 2016, Vol. 27
2016, Vol. 27 


