b National Institutes for Food and Drug Control, Beijing 100050, China ;
c Shaanxi Momentum Pharmaceutical Co., LTD, Xi'an 710075, China
Plumulanelumbinis (called “Lianzixin” in Chinese), is the green embryo of mature seed of Nelumbonucifera Gaertn. It has been used as a traditional Chinese medicine for hundreds of years for treating heart palpitations, aiding tranquillity and reducing blood pressure [1]. Earlier phytochemical studies revealed that this herbal medicine is rich in alkaloids, including bisbenzylisoquinoline, benzylisoquinoline, aporphine, and proaporphinealkaloids [2-5]. The pharmacological studies showed that these alkaloids have extensive biological activities such asantihypertension [5], hypoglycemic effect [6, 7], butyrylcholinesterase (BChE) inhibition [8], anti-pulmonaryfibrosis [9], anti-human immunodeficiency virus (HIV) activity [10], antiproliferative effect on CASMCs induced by phenylephrine [11], and potenitiation of vincristine-induced apoptosis in the human mammary MCF-7 multidrug-resistant cells [12].
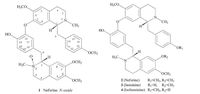
In this study, chemical investigations on Plumulanelu mbinis led to the isolation of one new bisbenzylisoquinoline alkaloid, neferine N-oxide (1), along with three known ones, neferine (2) [13], liensinine (3) [14], and isoliensinine (4) [3] (Fig. 1). Herein, we describe the isolation, structure elucidation, and the antioxidative activities of these compounds.
|
Download:
|
| Figure 1. Chemical structures of compounds 1-4. | |
2. Experimental 2.1. General experimental procedures
UV spectra were recorded on a JASCO (UV/VIS) V-550 spectrophotometer and IR spectra on a JASCOFT/IR480PLUS Fourier transform spectrophotometer with pressed KBr pellets in the range 200-4000 cm-1. Optical rotations were measured on a JASCO P- 1020 digital polarimeter and CD spectra were recorded on a JASCO 810 spectropolarimeter. NMR data were obtained on a Bruker A- 400 spectrometer, with TMS as an internal standard. Mass spectra were obtained with a Bruker Esquire 2000 spectrometer, and HRESI- MS were taken on a Micromass Q-TOF mass spectrometer. HPLC analyses were carried out on an SHIMADZU LC 20 AT/LC-6AD Series instrument equipped with an PDA detector, using an analytical 5 C18-MS-II (4.6 mm×250 mm, 5 μm, cosmosil) or a preparative 5 C18-MS-IIC18 (10 mm×250 mm, 5 μm, cosmosil) Column chromatography (CC) was conducted using Silica gel (Qingdao Haiyang Chemical Group Co., Qingdao, China), ODSA120- S150 (YMC Co., Ltd, made in Komatsu, Japan), and Sephadex LH-20 (Amersham Biosciences, made in Uppsala, Sweden).
2.2. Plant materialPlumulanelumbiniswere collected in Chengdu, Sichuan Province, China and were identified by associate professor Sheng-Li Wei (School of Chinese Material Medica, Beijing University of Chinese Medicine). A voucher specimen (2014060511) is deposited in the School of Chinese Material Medica, Beijing University of Chinese Medicine.
2.3. Extraction and isolationPlumulanelumbinis(2 kg) were refluxed with 80% (v/v) EtOH (20 L, 3×2 h). The EtOH extract was concentrated in vacuo and the resulting residue (100 g) was dissolved in water and partitioned successively with EtOAc and water-saturated n-BuOH to give three parts, EtOAc (42 g), n-BuOH (21 g) and H2O (35 g) soluble parts. The EtOAc part was subjected to silica gel (200-300 mesh) column chromatography and eluted with a CH2Cl2-MeOH gradient to obtain fifteen fractions. Fraction 6 (CH2Cl2-MeOH 20:1 eluate, 6 g) was separated on an ODS column with MeOH-H2O (60:40, v/v), and semi-preparative reverse-phase HPLC (cosmosil, 10 mm×250 mm, flow rate 4 mL/min) with MeOH-H2O-triethylamine( TEA) (58:42:0.05, v/v/v) to give compounds 1 (8 mg) and 2 (22 mg). Fraction 7 (CH2Cl2-MeOH 10:1 eluate, 7.5 g) was further separated an ODS column with MeOH-H2O (65:35, v/v), and then passed through semi-preparative reverse-phase HPLC (cosmosil, 10×250 mm, flow rate 4 mL/min) with MeOH-H2O-TEA (65:35:0.05, v/v/v) to give compounds 3 (12 mg) and 4 (16 mg).
(1R, 1′R) NeferineN-oxide (1): Amorphous yellow powder; [a]D 26.3-15.7 (c 1.0, CHCl3); UV (CHCl3) λmax(loge e):243 (3.99), 284 (3.82) nm; CD (MeOH): 207 (De-15.7), 234 (De-11.5), 289 (De-1.1) nm; IR (KBr, cm-1): vmax 3436, 2925, 1656, 1612, 1506, 1462, 1251, 1193, 1110, 1030, 956, 620; (+)-ESIMS: m/z 641 [M + H]+; (+)-HRESIMS m/z 641.3223 [M + H]+(calcd. for C38H45N2O7, 641.3227); 1H NMR (400 MHz, CDCl3): Table 1; 13C NMR (100 MHz, CDCl3): Table 1.
|
|
Table 1 NMR spectroscopic data for compounds 1 and 2a. |
(1R, 1′R) Neferine (2): Amorphous yellow powder; [a]D 26.3-33.8 (c 1.0, CHCl3). UV (CHCl3) λmax(loge e):243 (5.00), 285 (4.88) nm; CD (MeOH): 208 (De-23.7), 235 (De-12.8), 291 (De-2.9) nm; IR (KBr, cm-1): vmax 3432, 2925, 2854, 1652, 1638, 1545, 1558, 1105, 1035, 621; (+)-ESIMS:m/z 625 [M+ H]+; 1H NMR (400 MHz, CDCl3): Table 1; 13C NMR (100 MHz, CDCl3): Table 1.
2.4. Oxygen radical absorbance capacity (ORAC) assayThe ORAC assay was performed according to the Zhou method with slight modifications [15]. The automated ORAC assay was carried out on a GENiosLuciferase-based microplatereader (Tecan Group Ltd, Ma¨nnedorf, Switzerland) with an excitation/emission filter pair of 485/527 nm. Sodium fluorescein was used as a fluorescence probe, and the reaction was initiated with the addition of AAPH. EGCG was used as a positive control. The results were calculated on the basis of the difference in the area under the fluorescence decay curve between the AAPH control and each sample. The ORAC values of tested samples were calculated as the relative values of the area under the fluorescence decay curve using Trolox as a standard, and expressed as micromoles of Trolox equivalents (TE) per micromoles of sample (mmol TE/mmol). All of the samples were analyzed in quadruplicate.
3. Results and discussionCompound 1 was obtained as amorphous yellow powder [a]D 26.3-15.7 (c 1.0, CHCl3). The molecular formula was determined as C38H44N2O7 by HR-ESIMS at m/z 641.3223[M + H]+ (calcd. for C38H45N2O7, 641.3227) (Fig. S1 in Supporting information), with 18 degrees of unsaturation. Its IR spectrum (Fig. S3 in Supporting information) displayed absorption bands characteristic of hydroxy (3436 cm-1), methyl group (2925 and 1462 cm-1), aromatic ring (1656, 1612, and 1506 cm-1), and N→O (956 cm-1) functionalities [16].
The 1H NMR spectrum of 1 (Table 1) (Fig. S4 in Supporting information) exhibited two N-methylgroups at δ 3.18 (s, 3H, NCH3- 20) anδ 2.46 (s, 3H, NCH3-2), an aromatic AA′XX′ spin system at δ 6.90 (m, 2H, H-11, 15) anδ 6.67 (m, 2H, H-12, 14), an AMX spin system at δ 6.87 (d, 1H, J = 8.4 Hz, H-14′), 6.77 (dd, 1H, J = 8.4, 1.6 Hz, H-150) anδ 6.55 (d, 1H, J = 1.6 Hz, H-110), four singlets due to aromaticprotons at δ 6.61 (s, 1H, H-5), 6.40 (s, 1H, H-8), 6.53 (s, 1H, H-50), 6.23 (s, 1H, H-80), and four methoxy signals at δ 3.79 (s, 3H, OCH3-60), 3.75 (s, 3H, OCH3-6), 3.70 (s, 3H, OCH3-13), anδ 3.44 (s, 3H, OCH3-70).
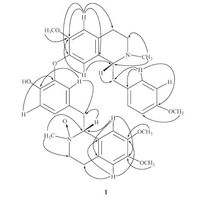
The 13C NMR spectrum (Table 1) (Fig. S5 in Supporting information) showed signals for 38 carbons, including thirteenquaternary sp2 carbons at δ 157.9, 149.1, 148.6, 147.6, 147.0, 145.9, 143.0, 131.7, 131.2, 130.7, 128.5, 125.6, and 122.3; eleven tertiary sp2 carbons at δ 130.6 (-2), 124.5, 120.1, 118.6, 117.0, 113.6 (-2), 112.7, 111.0, and 110.8; four methylene carbons at δ 63.0, 47.4, 26.3, 26.0; four methanecarbons at d 78.3, 64.6, 39.8, 37.4; four methoxylcarbons at d 56.0 (-2), 55.8, and 55.3; two Nmethylcarbons at d 53.1 and 42.9. With the aid of HSQC (Fig. S7 in Supporting information) and HMBC experiment (Fig. 2) (Fig. S8 in Supporting information), the chemical shifts of carbons connected with protons were confirmed. The planar structure was confirmed by key HMBC correlations (Fig. 2). The NMR spectrum of 1 resembled those of neferine (2), one of the main alkaloids of this plant, except for the chemical shifts corresponding to carbons and protons around one of the methyl singlet (Table 1). In the 1H NMR spectrum, there were marked downfield chemical shift of a methyl singlet at δ 3.18, as well as the significant downfield signals at δ 4.53 for H-10, δ 3.62 for H-30 , δ 3.86 for H-90, δ 6.23 for H-80 . In the 13C NMR spectrum, there were also marked downfield chemical shift of a methyl singlet at d 53.1, as well as the significant downfield signals at δ 78.3 for C-10 , δ 63.0 for C-30 . All the differences indicated the presence of an electron-withdrawing group bonded to 20-N-methyl. The molecular weight of compound 1 was just 16 more than that of neferine (2), indicated that compound 1 is the N-oxide derivative of neferine.
|
Download:
|
| Figure 2. Key HMBC correlations of compound 1. | |
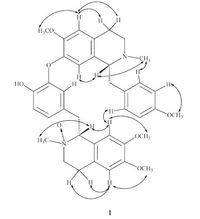
In the NOESY spectrum (Fig. S9 in Supporting information), important NOESY cross peaks between H-5 and the methoxy signal at δ 3.75, between H-12/14 and the methoxy signal at δ 3.70, between H-50 and the methoxy signal at δ 3.79, and between H-80 and the methoxy signal at δ 3.44, indicated the presence of methoxy groups at C-6, C-13, C-6′and C-7′ (Fig. 3). NOESY interactions, H-1 and H-10with δ 2.46 and δ 3.18, respectively, revealed a syn-periplanar relationship between H-1, H-1′ and their vicinal N-methyl group.
|
Download:
|
| Figure 3. Selected NOESY correlations of compound 1. | |
N. Katsumi et al. [10] synthesized the four stereoisomers of neferine and evaluated their opticalrotation. The optical rotation of compound 2 showed [a]D 26.3-33.8 (c 1.0, CHCl3), which was identical with that of (1R, 1′R)neferine [a]D 28 -37.8 (c1.43, CHCl3) [10]. Thus, compound 2 was assigned as (1R, 1′R)neferine.
The CD spectrum of 1 showed negative Cotton effects at 207 nm(Δε- 15.7), 234 nm (Δε- 11.5), and 289 nm (Δε- 1.1); The CD spectrum of 2 showed negative Cotton effects at 208 nm (Δε- 23.7), 235 nm(Δε- 12.8), and 291 nm(Δε- 2.9). The CD spectrum of 1 is similar to that observed in compound 2 (1R, 1′R)neferine (Fig. 4). Therefore, C-1, C-1′ of compound 1 was both assigned the R configurations. Thus, compound 1 is a new compound and elucidated as (1R, 1′R) neferine N-oxide, and the CD spectrum of (1R, 1′R)neferine is first reported.

|
Download:
|
| Figure 4. CD spectra of 1 and 2 in CH3OH. | |
The antioxidant activities of compounds 1-4 were assessed using the ORAC assay with EGCG as the positive control. Fig. 5 demonstrates that all these compounds showed the different levels of oxygen radical absorbance capacity.

|
Download:
|
| Figure 5. Antioxidant capacity of compounds 1-4 in vitro evaluated by the ORAC method. Each value was expressed as means ±SD, n = 4. | |
4. Conclusion
Four bisbenzylisoquinoline alkaloids including one new compound, neferine N-oxide (1) and three known compounds, neferine (2), liensinine (3), and isoliensinine (4), were isolated from Plumulanelumbinis. Compound 1 is a new naturally occurring Noxide belonging to the bisbenzylisoquinoline alkaloid class, and the CD spectrum of (1R, 1′R) neferine was first reported. The four compounds displayed different antioxidant activities.
Acknowledgment This project was financially supported by 12th Five-Year Plan Major New Drug Discovery Science and Technology Projects for support of this research (No. 2014ZX09304307-002).| [1] | Pharmacopoeia Commission of China, Chinese Pharmacopoeia, 1, Chemical Industry Press, Beijing, 2015, pp. 273-274. |
| [2] | Lin Z.T, Yang R.N, Guan Z, Chen A.L, Li W, Ultra-performance LC separation and quadrupole time-of-flight MS identification of major alkaloidsin plumula nelumbinis. Phytochem. Anal 25 (2014) 485–494. DOI:10.1002/pca.v25.6 |
| [3] | Yang J, Zhou K.L, NMR spectroscopic analysis of neferine and isoliensinine. Magn. Reson. Chem 42 (2004) 994–997. DOI:10.1002/(ISSN)1097-458X |
| [4] | Itoh A, Saitoh T, Tani K, Bisbenzylisoquinoline alkaloids from Nelumbonucifera. Chem. Pharm. Bull 59 (2011) 947–951. DOI:10.1248/cpb.59.947 |
| [5] | Nishibe S, Tsukamoto H, Kinoshita H, Kitagawa S, Sakushima A, Alkaloids from embryo of the seed of Nelumbonucifera. J. Nat. Prod 49 (1986) 547–548. |
| [6] | Hu W.S, Guo L.J, Feng X.L, Jiang M.X, Hypotensive effects of neferine. Chin. J. Pharmacol. Toxicol 4 (1990) 107–110. |
| [7] | Zhang M, Chen L, Berberine in type 2 diabetes therapy:a new perspective for an old antidiarrheal drug?. Acta Pharm. Sinica B2 (2012) 379–386. |
| [8] | Lin Z.T, Wang H, Fu Q.R, Simultaneous separation, identification and activity evaluation of three butyrylcholinesterase inhibitors from Plumula nelumbinis using on-line HPLC-UV coupled with ESI-IT-TOF-MS and BChE biochemical detection. Talanta 110 (2013) 180–189. DOI:10.1016/j.talanta.2013.02.033 |
| [9] | Xiao J.H, Zhang J.H, Chen H.L, Feng X.L, Wang J.L, Inhibitory effects of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. Planta Med 71 (2005) 225–230. DOI:10.1055/s-2005-837821 |
| [10] | Kashiwada Y, Aoshima A, Ikeshiro Y, Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbonucifera, and structure-activity correlations with related alkaloids. Bioorg. Med. Chem 13 (2005) 443–448. DOI:10.1016/j.bmc.2004.10.020 |
| [11] | Xiao J.H, Zhang Y.L, Ding L.L, Feng X.L, Wang J.L, Effects of isoliensinine on proliferation of porcine coronary arterial smooth muscle cells induced by phenylephrine. Acta Pharm. Sinica 40 (2005) 105–110. |
| [12] | Ye G.Z, Wang J.H, Sun A.X, Poteintation of vincristine-induced apoptosis by tetrandrine, neferine and dauricine in the human mammary MCF-7 multidrugresistant cells. Acta Pharm. Sinica 36 (2001) 96–99. |
| [13] | Nishimura K, Horii S, Tanahashi T, Sugimoto Y, Yamada J, Synthesis and pharmacological activity of alkaloids from embryo of Lotus, Nelumbonucifera. Chem. Pharm. Bull 61 (2013) 59–68. DOI:10.1248/cpb.c12-00820 |
| [14] | Wu J.Z, Yuan H.L, Wang J.L, Sun H.D, Spectroscopic elucidation of liensinine. Chin. Tradit. Herbal Drugs 29 (1998) 364–367. |
| [15] | Zhou Z.Q, Fan H.X, He R.R, Lycibarbarspermidines A-O, new dicaffeoylspermidine derivatives from wolfberry, with activities against Alzheimer's disease and oxidation. J. Agric. Food Chem 64 (2016) 2223–2237. DOI:10.1021/acs.jafc.5b05274 |
| [16] | Ding P.L, Huang H, Zhou P, Chen D.F, Quinolizidine alkaloids with anti-HBV activity from Sophoratonkinensis. Planta Med 72 (2006) 854–856. DOI:10.1055/s-2006-946639 |
 2016, Vol. 27
2016, Vol. 27 



