b Department of Chemistry, PSG College of Technology, Peelamedu, Coimbatore-641004, Tamilnadu, India
One of the main aspects of contemporary chemistry deals with use of recyclable catalyst to enhance sustainability of a chemical process without compromising the efficiency [1-3].In this context magnetically separable catalyst are significant due to their ease of separation and purification [4-6].Room temperature ionic liquid (RTIL) have received much attention due to their negligible volatility, non-inflammability, thermal stability, easy recyclability, broad liquid temperature range and the ability to dissolve wide range of inorganic and organic compounds [7-12].Combination of imidazole or benzimidazole cation with tribromide ion results in the formation of so called ionic liquid tribromide (IL-Br3) [10, 13].IL-Br3 have recently been proved to be efficient catalysts and reagents for variety of reaction.In spite of several advantage of using ILs, they have some problems when used as reaction medium (1) usually expensive even though commercially available (2) required immiscible solvent to separate organic product from IL-catalyst.Thus IL based compounds although have shown great potential as homogeneous catalysts in laboratory scale, they have not been used in large scale industrial application in catalysis.Heterogeneous catalysts although important from sustainability point of view, they usually have lower activity and selectivity as compound to their homogeneous counterparts.However these small problems of heterogeneous catalysts can be removed if the size of the catalyst is reduced to nanoscale.In fact nanoparticles are referred to as "quasi-homogeneous" support that bridges the gap between homogeneous and heterogeneous catalysis, reaping the benefit of both the systems [14, 15].The advantage of heterogeneous nano catalyst can be increased many fold if they are magnetically recoverable.Therefore design of an ionic liquid based catalyst immobilized on magnetic nanoparticles (MNP) would assume greatest significance in term of their efficiency and recyclability.
Multicomponent reaction (MCR) [16-19] has emerged as a powerful tool in organic synthesis due to their ability to prepare the target molecule of structural complexity in a single step.Moreover MCR offer more advantages such as simplicity, synthetic efficiency and atom economy over conventional chemical reaction [20-22].Piperidine and its derivatives are considered to be extremely important in heterocyclic and pharmaceutical chemistry.Natural products possessing piperidine scaffold have been reported to exhibit antibacterial, antihypertensive, anti-inflammatory and anti-convulsant activities [23-29]. First report on MCR between amine, aldehyde and 1-3-dicarbonyl to synthesize functionalized piperidine appeared in 1943 [30].Recently a variety of organic catalysts have been introduced which effectively catalyze synthesis of functionalized piperidine through MCR.Some of the important one are wet picric acid [31], p-toluene sulfonic acid [32], bromodimethylsulfonium bromide (BDMS) [33], tetrabutyl ammonium tribromide (TBATB) [34], iodine (I2) [35].Recently IL L-proline nitrate has been reported as green catalyst for synthesis of piperidine derivatives through MCR [36].However in spite of having many advantages all such catalyst has one or more drawbacks such as inefficient and cumbersome recovery and reuse, use of quaternary nitrogen based compounds etc.These drawbacks pose some concerns on the sustainability aspect of the protocol.
As a part of our ongoing research endeavour [4, 37-43], on synthesis and application of tribromide and nano catalysts, we wish to report herein synthesis of novel IL-Br3 catalyst supported on Fe2O3 NPs, and its catalytic activity on the synthesis of functionalized piperidine through MCR of amine, aldehyde and 1, 3-dicarbonyl.
2. ExperimentalSynthesis of α-Fe2O3 nanoparticles: In a typical procedure, 8.08 g of Fe(NO)3·9H2O (20 mmol) were dissolved in minimum volume of distilled water.The pH was adjusted to~9 by drop wise addition of NH4OH solution.Subsequently, 0.68 g CTAB solution was added to the mixed solution.After 24 h of continuous stirring, the product was washed with ethanol and dried at 110 ℃ oven for 12 h, followed by calcination at 500 ℃ for 6 h in the presence of helium (10 mL min-1).Here CTAB acts as a capping agent in controlling the size of Fe2O3 NPs.
Preparation of Fe2O3 coated by (3-chloropropyl)-triethoxysi-lane (ClPr-Si@Fe2O3): α-Fe2O3NPs (6 g) synthesized with present method was dispersed in 250 mL ethanol/water (volume ratio 1:1) solution under sonication for 30 min, and then CPTES (15 mL) was added to the mixture and stirred for 24 h at room temperature.The resulting product was separated by an external magnet and were washed 4 times with ethanol and dried under vacuum.
Preparation of 3-(1-benzimidazole)propyltriethoxysilane functionalized nanoFe2O3 ((3-(1-benzimidazole)Pr-Si@Fe2O3): A sodium salt of benzimidazole was prepared by reacting 50% sodium hydride (0.479 g) with benzimidazole (1.17 g) with in mineral oil in dry toluene (15 mL) under inert atmosphere for 3 h.The resultant solution of sodium benzimidazole was mixed with ClPr-Si@Fe2O3 (3 g) and refluxed for 24 h.After cooling the mixture to room temperature the product was separated by magnetic decantation.The separated product was washed separately with acetone and methanol to remove the unreacted productand dried under vacuum.
Preparation of benzimidazole tribromide supported on 3-(1-benzimidazole)Pr-Si@Fe2O3 (Fe2O3-BIM tribromide): 3-(1-Benzimidazole)Pr-Si@Fe2O3 (3 g) was stirred in chloroform (10 mL), to that a solution of bromine (0.40 g) in dry chloroform (5 mL) was added drop wise and the mixture was stirred at ice cold condition for 5 h.The product was separated by magnetic decantation and was washed several times with chloroform and stored in refrigerator until further use.
General procedure for the synthesis of highly functionalized piperidines: To a solution of methyl acetoacetate (1 mmol) and amine (2 mmol) and in 5 mL of ethanol, 10 mol % of catalyst was added and stirred at room temperature.After 20 min, aromatic aldehyde (2 mmol) was added and the reaction mixture was stirred further till a thick precipitate of the product was obtained.The catalyst was then separated magnetically from the reaction mixture.The solid product was filtered off and washed with ethanol.
3. Results and discussion 3.1. Synthesis of the IL-Br3 catalystNano Fe2O3-BIM tribromide was prepared according to the procedure shown in Scheme 1.α-Fe2O3 NPs was synthesized using Fe(NO)3·9H2O, CTAB and NH4OH, where CTAB acted as a capping agent in controlling the size of α-Fe2O3 nanoparticles.Subsequently the as synthesized NPs were grafted with (3-chloropro-pyl)-triethoxysilane through covalent bonding.The CPTES grafted α-Fe2O3NPs was further reacted with sodium salt of benzimidazole in dry toluene under refluxed condition for 24 h to produce 3-(1-benzimidazole)propyltriethoxysilane functionalized α-Fe2O3-magnetic NPs (3-(1-benzimidazole)Pr-Si@Fe2O3).Finally 3-(1-benzimidazole) Pr-Si@Fe2O3 was converted to corresponding tribromide by addition of liquid bromine in dry chloroform.The as-synthesized IL tribromide was used as catalyst for one-pot synthesis of highly substituted piperidines Scheme 2.

|
Download:
|
| Scheme. 1. Scheme represent the formation of ionic liquid tribromide. | |
|
Download:
|
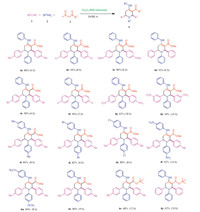
| Scheme. 2. Scheme for the synthesis of functionalized piperidines and scope of Fe2O3-BIM tribromide catalyzed five component reactions for the synthesis of functionalized piperidines. | |
3.2. Catalyst characterization
All the intermediates as well as the final tribromide catalyst were studied by FTIR (Fig.S1 in Supporting information).All spectra showed a characteristic broad band around 3420 cm-1 and 1640 cm-1 respectively, which may be attributed to stretching and bending mode of Si-OH group and adsorbed water.Moreover bands at 589 cm-1 and 467 cm-1correspond to Fe-O vibration of the iron oxide core.The ClPr-Si@Fe2O3 (Fig S1a) shows a absorption peak at 952 cm-1 assigned as Fe-O-Si absorption band which confirm that CPTES is successfully grafted on the surface of Fe2O3.Fig S1b and c) are spectra of 3-(1-benzimidazole)Pr-Si@Fe2O3 and Fe2O3-BIM tribromide, respectively.It can be seen that additional peaks appeared at 3112, 2960 1547, and 1382 cm-1.These peaks may be attributed to C-H stretching vibration of benzimidazole moiety, alkyl chain starching, and vibration of C=N and C=C of the benzimidazole ring, respectively.Further Fe2O3-BIM tribromide exhibits a characteristic broad band at 993 cm-1 which was not observed at 3-(1-benzimidazole)Pr-Si@Fe2O3.The formation of tribromide (Br3-) was also confirmed by UV visible spectroscopy which shows an intense UV absorption at 272 nm (Fig.S2 in Supporting information).
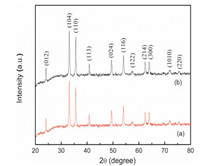
The powder XRD patterns were recorded for identification of phases exhibited by the synthesized materials.Fig. 1a showed the powder XRD patterns of the synthesized Fe2O3 catalyst. The diffraction peaks match well with the reported data of α-Fe2O3 (JCPDS File No. 87-1166). α-Fe2O3 possess a rhombohedrally centered hexagonal structure of the corundum type with a close-packed oxygen lattice in which two thirds of the octahedral sites are occupied by Fe(Ⅲ) ions [4, 44].The XRD pattern of Fe2O3-BIM tribromide (Fig. 1b) is consistent with standard data pattern of Fe2O3 confirming retention of the crystallinity during the functionalization process.The average crystallite sizes of the synthesized Fe2O3 and Fe2O3-BIM tribromide were estimated by the Debye-Scherrer equation [4].The average crystallite sizes of were found to be 15.1 and 21.7 nm for Fe2O3 and Fe2O3-BIM tribromide, respectively.
|
Download:
|
| Figure 1. Powder XRD patterns of (a) Fe2O3 nanoparticles and (b) Fe2O3-BIM tribromide. | |
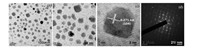
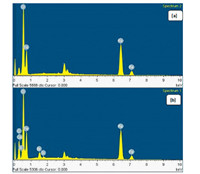
The TEM image of Fe2O3-BIM tribromide nanomaterial (Fig. 2) showed mostly irregular shapes of the particles of Fe2O3 with sizes around 5-80 nm.The spacing of the lattice fringes were found to be separated by 0.271 nm possibly due to (104) plane of Fe2O3.Electron diffraction (ED) pattern indicated single crystalline nature of the material.The EDAX patterns Fig. 3a, b of Fe2O3 and Fe2O3-BIM tribromide shows the presence of element composition.Fig. 3a indicated presence of Fe and O in the synthesized materials.Fig. 3b shows the EDX spectrum of the catalyst (Fe2O3-BIM tribromide) indicates the presence of Fe, O, Si, C, N and Br which confirm the formation of Fe2O3-BIM tribromide. The field emission scanning electron microscopy (FE-SEM) image of Fe2O3-BIM tribromide is shown in Fig. 4.The figure displays uniform nano sized particles of sizes 50-80 nm.It is clearly evident form the image that all the nanoparticles are surrounded by benzimidazole molecules.It can be clearly seen from the fig that the white organic BIM is coated on the surface of Fe2O3.Fig.S3a, b in Supporting information represents TGA curves for ClPr-Si@Fe2O3 and Fe2O3-BIM tribromide, respectively.The TGA studies were mainly made to determine the percentage loss of organic functional groups chemisorbed on the surface of nano Fe2O3.From Fig S3a we can observe, that decomposition of the organic groups takes place at a temperature of above of 200 ℃.A weight loss of about 13% was observed in the range of 150-350 ℃ which is attributed to the removal of (3-chloropropyl)-triethox-ysilane from the surface of Fe2O3.However Fe2O3-BIM tribromide showed a decomposition pathway in the range of 200 -500 ℃.The weight loss of 65% can be attributed to removal of adsorbed solvents or trapped water from the catalyst and due to breakdown of the benzimidazole tribromide moieties.On the ground of the TGA data we can say that the catalyst is stable at a temperature up to 200 ℃ and we can safely performed all the catalytic reaction within this temperature.
|
Download:
|
| Figure 2. (a, b) TEM images (c) HRTEM image and (d) ED pattern of Fe2O3-BIM tribromide. | |
|
Download:
|
| Figure 3. The EDX pattern of (a) Fe2O3nanoparticles and (b) Fe2O3-BIM tribromide. | |

|
Download:
|
| Figure 4. The SEM image of Fe2O3-BIM tribromide. | |
The magnetic hysteresis measurements (Fig. 5a, b) of Fe2O3 and Fe2O3-BIM tribromide were carried out at 300 K with the applied magnetic field sweeping from -20000 G to +20000 G.The saturation magnetization values of the samples (Fe2O3 and Fe2O3-BIM tribromide) are reduced from 9.47 emug-1 to 8.59 emug-1.The decrease in the saturation magnetization of Fe2O3-BIM tribromide was due to immobilization of BIM tribromide on the surface of Fe2O3NPs.However, magnetization value of the catalysts is good enough to enable magnetic separation the catalyst from the reaction mixture using an external magnetic field.The magnetic hysteresis loops showed a super paramagnetic behavior.

|
Download:
|
| Figure 5. VSM of (a) Fe2O3 nanoparticles, (b) Fe2O3-BIM tribromide and (c) N2 adsorption-desorption isotherms of Fe2O3-BIM tribromide. | |
N2 adsorption-desorption measurements were conducted to determine the Brunauer Emmett Teller (BET) specific surface area of Fe2O3-BIM tribromide NPs. High specific surface area of 462.42 m2 g-1 and pore volume of 0.923 cm3 g-1 was observed. The high specific surface area of the catalysts can be corelated to efficiency of its perofrmance.The amount of N2 adsorbed rose gradually at lower relative pressures and then increased sharply at higher relative pressures as shown in (Fig. 5c).This follows the characteristics of a type Ⅳ isotherm with a type H3 hysteresis loop associated with aggregates of plate-like particles forming slit-like pores [45].The observed hysteresis loop shifts to a higher relative pressure on approaching P/P0=1, suggesting the presence of macropores and a low degree of aggregation [45].The Barrett Joyner Halenda (BJH) pore size distribution indicated that most of the pores range from 2 nm to 14 nm as shown in the inset of (Fig. 5c), which further confirmed the loose structures and a low degree of aggregation.
3.3. Catalytic activity of nano Fe2O3-BIM tribromide for the synthesis of functionalized piperidines by one-pot multicomponent reaction (MCRs).After successful synthesis and characterization of Fe2O3-BIM tribromide, we examined its catalytic activity for the synthesis of functionalized piperidines.At the beginning of the catalytic test, a mixture of 4-methylbenzaldehyde (2 mmol), methyl acetoacetate (1 mmol) and aniline (2 mmol) in acetonitrile (5 mL) was treated with 10 mol% of catalyst at room temperature.The reaction was carried out for nearly 8 hours to afford a solid precipitate which was filtered and washed with ethanol to give functionalized piperidine 1a in 82% yield.For optimization of the catalyst and solvent, several trial reactions were performed under same reaction condition.The results are presented in Table S1 in Supporting information.
Increase in the amount of the catalyst beyond 10 mol % did not improve the yield of the product to any noticeable extent.However, lower loading of the catalyst significantly reduces the yield when the reaction was carried out for the same reaction time.It was also observed that ethanol is the best solvent in terms of yield and reaction time.Using these optimized conditions several functionalized piperidines were synthesized by using different combination of aldehydes, aniline and 1, 3-dicarbonyl compounds.The results are summarized in Scheme 2.
After competition of the reaction, the solid catalyst was recovered magnetically from the reaction mixture and washed thoroughly with acetone and reused in next cycles under similar reaction conditions as to check the efficiency of the catalyst.The solid product of piperidine was filtered off and washed with ethanol.The activity of the recovered catalyst after 5 consecutive run did not lead to any significant decline in the catalytic activity in terms of yield (Fig.S4 in Supporting information).
4. ConclusionIn conclusion, we have achieved preparation of new ionic liquid tribromide catalyst immobilized on a nano Fe2O3.The synthesis of α-Fe2O3 was based on precipitation of iron (Ⅲ) followed by calcination at 500 ℃.This novel nano catalyst was characterized by different physical techniques.All the analytical results unambiguously indicate the formation desired nano Fe2O3 supported benzimidazolium tribromide.The as-synthesized immobilized tribromide has been used as magnetically recoverable heterogeneous catalyst for synthesis of functionalized piperidines through three-component reaction between aldehyde, amine and 1, 3-dicarbonyl compounds.This catalytic MCR procedure provides very high yields of the product in reasonably short reaction times.The catalyst can be recovered by magnetic decantation and used up to 5 times without significant loss in activity.
AcknowledgmentThe authors thank SAIF, IIT Madras, SAIF, IIT Bombay and SAIF, North Eastern Hill University for analysis.The Director NIT Silchar is acknowledged for financial support (STIS project sanction No.PA/254/23093-96).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.005.
| [1] | F. Shi, M.K. Tse, M.M. Pohl, Tuning catalytic activity between homogeneous and heterogeneous catalysis: improved activity and selectivity of free nano-Fe2O3 in selective oxidations. Angew. Chem. Int. Ed. 46 (2007) 8866–8868. DOI:10.1002/(ISSN)1521-3773 |
| [2] | A.C. Garade, M. Bharadwaj, S.V. Bhagwat, A.A. Athawale, C.V. Rode, An efficient γ-Fe2O3 catalyst for liquid phase air oxidation of p-hydroxybenzyl alcohol under mild conditions. Catal. Commun. 10 (2009) 485–489. DOI:10.1016/j.catcom.2008.10.044 |
| [3] | H.N. Dadhania, D.K. Raval, A.N. Dadhania, Magnetically retrievable magnetite (Fe3O4) immobilized ionic liquid: an efficient catalyst for the preparation of 1-carbamatoalkyl-2-naphthols. Catal. Sci. Technol. 5 (2015) 4806–4812. DOI:10.1039/C5CY00849B |
| [4] | B. Paul, B. Bhuyan, D.D. Purkayastha, S.S. Dhar, Facile synthesis of α-Fe2O3 nanoparticles and their catalytic activity in oxidation of benzyl alcohols with periodic acid. Catal. Commun. 69 (2015) 48–54. DOI:10.1016/j.catcom.2015.05.017 |
| [5] | T.K. Kundu, M. Mukherjee, D. Chakravorty, T.P. Sinha, Growth of nano-α-Fe2O3 in a titania matrix by the sol-gel route. J. Mater. Sci. 33 (1998) 1759–1763. DOI:10.1023/A:1004376515384 |
| [6] | M.A. Bhosale, D. Ummineni, T. Sasaki, D. Nishio-Hamane, B.M. Bhanage, Magnetically separable γ-Fe2O3 nanoparticles: an efficient catalyst for acylation of alcohols, phenols, and amines using sonication energy under solvent free condition, J. Mol. Catal. A Chem. 404- 405 (2015) 8–17. |
| [7] | P.M. Leó, C. Morin, C. Philouze, Structure revision of medermycin/lactoquinomycin A and of related C-8 glycosylated naphthoquinones. Org. Lett. 4 (2002) 2711–2714. DOI:10.1021/ol026222e |
| [8] | K. Tatsuta, H. Ozeki, M. Yamaguchi, M. Tanaka, T. Okui, Enantioselective total synthesis of medermycin (lactoquinomycin). Tetrahedron Lett. 31 (1990) 5495–5498. DOI:10.1016/S0040-4039(00)97881-X |
| [9] | H.R. Shaterian, K. Azizi, Acidic ionic liquids catalyzed one-pot, pseudo fivecomponent, and diastereoselective synthesis of highly functionalized piperidine derivatives, J. Mol. Liq. 180 (2013) 187–191. DOI:10.1016/j.molliq.2013.01.020 |
| [10] | H. Veisi, A. Sedrpoushan, P. Mohammadi, A.R. Faraji, S. Sajjadifar, A new recyclable 1, 4-bis(3-methylimidazolium-1-yl)butane ditribromide[bMImB] (Br3)2 ionic liquid reagent for selective bromination of anilines or phenols and a-bromination of alkanones under mild conditions. RSC Adv. 4 (2014) 25898–25903. DOI:10.1039/c4ra03006k |
| [11] | J.P. Hallett, T. Welton, Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem. Rev. 111 (2011) 3508–3576. DOI:10.1021/cr1003248 |
| [12] | T. Welton, Room-temperature ionic liquids, Solvents for synthesis and catalysis. Chem. Rev. 99 (1999) 2071–2084. DOI:10.1021/cr980032t |
| [13] | S. Otokesh, E. Kolvari, A. Amoozadeh, N. Koukabi, Magnetic nanoparticle-supported imidazole tribromide: a green, mild, recyclable and metal-free catalyst for the oxidation of sulfides to sulfoxides in the presence of aqueous hydrogen peroxide. RSC Adv. 5 (2015) 53749–53756. DOI:10.1039/C5RA07530K |
| [14] | R. Giernoth, Task-specific ionic liquids. Angew. Chem. Int. Ed 49 (2010) 2834–3839. DOI:10.1002/anie.200905981 |
| [15] | S.G. Lee, Functionalized imidazolium salts for task-specific ionic liquids and their applications. Chem. Commun. (2006) 1049–1063. |
| [16] | J. Zhu, H. Bienayme, Multicomponent Reactions-Superior Chemistry Technology for the New Millennium, Weinheim: Wiley, 2005 . |
| [17] | I. Ugi, Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 73 (2001) 187–192. |
| [18] | D.J. Ramón, M. Yus, Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew. Chem. Int. Ed. 44 (2005) 1602–1634. DOI:10.1002/anie.200460548 |
| [19] | A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106 (2006) 17–89. DOI:10.1021/cr0505728 |
| [20] | X.Y. Zhang, X.Y. Li, X.S. Fan, A novel synthesis of pyrazolo [3, 4-b]pyridine derivatives through multi-component reaction in ionic liquid. Chin. Chem. Lett. 19 (2008) 153–156. DOI:10.1016/j.cclet.2007.12.009 |
| [21] | A. Dömling, I. Ugi, Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39 (2000) 3168–3210. DOI:10.1002/(ISSN)1521-3773 |
| [22] | B.M. Trost, Atom economy-a challenge for organic synthesis: homogeneous catalysis leads the way. Angew. Chem. Int. Ed. 34 (1995) 259–281. DOI:10.1002/(ISSN)1521-3773 |
| [23] | S.W. Pelletier, Alkaloids: Chemical and Biological Perspectives, New York: John Wiley & Sons, 1987 . |
| [24] | J.W. Daly, T.F. Spande, H.M. Garraffo, Alkaloids from amphibian skin: a tabulation of over eight-hundred compounds. J. Nat. Prod. 68 (2005) 1556–1575. DOI:10.1021/np0580560 |
| [25] | P.S. Watson, B. Jiang, B. Scott, A diastereoselective synthesis of 2, 4-disubstituted piperidines: scaffolds for drug discovery. Org. Lett. 2 (2000) 3679–3681. DOI:10.1021/ol006589o |
| [26] | S. Petit, J.P. Nallet, M. Guillard, Synthèses et activités psychotropes de 3. 4-diarylpipéridines. Corrélation structure-activite et recherche d'une activité antihypertensive. Eur. J. Med. Chem. 26 (1991) 19–32. DOI:10.1016/0223-5234(91)90209-6 |
| [27] | Y.F. Zhou, V.E. Gregor, B.K. Ayida, Synthesis and SAR of 3, 5-diaminopiperidine derivatives: novel antibacterial translation inhibitors as aminoglycoside mimetics. Bioorg. Med. Chem. Lett. 17 (2007) 1206–1210. DOI:10.1016/j.bmcl.2006.12.024 |
| [28] | M. Misra, S.K. Pandey, V.P. Pandey, Organocatalyzed highly atom economic one pot synthesis of tetrahydropyridines as antimalarials. Bioorg. Med. Chem. 17 (2009) 625–633. DOI:10.1016/j.bmc.2008.11.062 |
| [29] | B. Ho, A.M. Crider, J.P. Stables, Synthesis and structure-activity relationships of potential anticonvulsants based on 2-piperidinecarboxylic acid and related pharmacophores. Eur. J. Med. Chem. 36 (2001) 265–286. DOI:10.1016/S0223-5234(00)01206-X |
| [30] | T. Boehm, W. Stöcker, Über die Bildung von γ-piperidonderivaten aus Azetessigester, aromatischen Aldehyden und Aminen, eine Modifikation der Hantzsch schen Pyridinsynthese, Arch. Pharm. 281 (1943) 62–77. |
| [31] | C. Mukhopadhyay, S. Rana, R.J. Butcher, A.M. Schmiedekamp, First report of syn isomers in the diastereoselective synthesis of highly functionalized piperidines catalysed by wet picric acid: factors influencing the syn-anti ratios. Tetrahedron Lett. 52 (2011) 5835–5840. DOI:10.1016/j.tetlet.2011.08.140 |
| [32] | S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, S.J. Shams-Najafi, One-pot multicomponent synthesis of highly substituted piperidines using p-toluenesulfonic acid monohydrate as catalyst. Monatsh Chem. 143 (2012) 939–945. DOI:10.1007/s00706-011-0671-7 |
| [33] | A.T. Khan, T. Parvin, L.H. Choudhury, Effects of substituents in the β-Position of 1, 3-dicarbonyl compounds in bromodimethylsulfonium bromide-catalyzed multicomponent reactions: a facile access to functionalized piperidines. J. Org. Chem. 73 (2008) 8398–8402. DOI:10.1021/jo8014962 |
| [34] | A.T. Khan, M. Lal, M.M. Khan, Synthesis of highly functionalized piperidines by one-pot multicomponent reaction using tetrabutylammonium tribromide (TBATB). Tetrahedron Lett. 51 (2010) 4419–4424. DOI:10.1016/j.tetlet.2010.06.069 |
| [35] | A.T. Khan, M.M. Khan, K.K.R. Bannuru, Iodine catalyzed one-pot five-component reactions for direct synthesis of densely functionalized piperidines. Tetrahedron 66 (2010) 7762–7772. DOI:10.1016/j.tet.2010.07.075 |
| [36] | N.R. Agrawal, S.P. Bahekar, P.B. Sarode, S.S. Zade, H.S. Chandak, L-Proline nitrate: a recyclable and green catalyst for the synthesis of highly functionalized piperidines. RSC Adv. 5 (2015) 47053–47059. DOI:10.1039/C5RA08022C |
| [37] | B. Paul, D.D. Purkayastha, S.S. Dhar, S. Das, S. Haldar, Facile one-pot strategy to prepare Ag/Fe2O3 decorated reduced graphene oxide nanocomposite and its catalytic application in chemoselective reduction of nitroarenes. J. Alloys Compd. 681 (2016) 316–323. DOI:10.1016/j.jallcom.2016.04.229 |
| [38] | B. Paul, B. Bhuyan, D.D. Purkayastha, S.S. Dhar, B.K. Patel, Hexamethonium bis(tribromide) (HMBTB) a recyclable and high bromine containing reagent. Tetrahedron Lett. 56 (2015) 5646–5650. DOI:10.1016/j.tetlet.2015.08.063 |
| [39] | R.R. Dey, B. Paul, S.S. Dhar, Novel metal-and mineral-acid-free synthesis of organic ammonium tribromides and application of ethylenephenanthrolium bistribromide for bromination of active methylene group of 1, 3-diketones and b-ketoesters. Synth. Commun. 45 (2015) 714–726. DOI:10.1080/00397911.2014.979509 |
| [40] | R.R. Dey, B. Paul, S.S. Dhar, S. Bhattacharjee, Novel protocol for the synthesis of organic ammonium tribromides and investigation of 1, 1'-(Ethane-1, 2-diyl)dipiperidinium bis(tribromide) in the silylation of alcohols and thiols. Chem. Lett. 43 (2014) 1545–1547. DOI:10.1246/cl.140564 |
| [41] | U. Bora, G. Bose, M.K. Chaudhuri, Regioselective bromination of organic substrates by tetrabutylammonium bromide promoted by V2O5 H2O2: an environmentally favorable synthetic protocol. Org. Lett. 2 (2000) 247–249. DOI:10.1021/ol9902935 |
| [42] | M.K. Choudhuri, U. Bora, S.K. Dehury, et al., Process for preparing quaternary ammonium tribromides, US 7005548B2. |
| [43] | B. Paul, B. Bhuyan, D.D. Purkayastha, S.S. Dhar, Green synthesis of silver nanoparticles using dried biomass of Diplazium esculentum (retz.) sw. and studies of their photocatalytic and anticoagulative activities. J. Mol. Liq. 212 (2015) 813–817. DOI:10.1016/j.molliq.2015.10.032 |
| [44] | R. Zboril, M. Mashlan, D. Petridis, Iron(Ⅲ) oxides from thermal processes-synthesis, structural and magnetic properties, mössbauer spectroscopy characterization, and applications. Chem. Mater. 14 (2002) 969–982. DOI:10.1021/cm0111074 |
| [45] | F.Z. Mou, J.G. Guan, Z.D. Xiao, Solvent-mediated synthesis of magnetic Fe2O3 chestnut-like amorphous-core/(-phase-shell hierarchical nanostructures with strong As(V) removal capability. J. Mater. Chem. 21 (2011) 5414–5421. DOI:10.1039/c0jm03726e |
 2016, Vol. 27
2016, Vol. 27 


