Macleaya cordata (Willd) R. Br. is a member of the Macleaya genus plants in the family of Papaveraceae, which is mainly distributed in North America, Europe, South and Northwest China. M. cordata has been widely used as a folk herbal medicine in China, North America, and Europe, where it is applied to cure cervical cancer and thyroid cancer, and to manage insect bites and ringworm infection [1-4]. Currently, M. cordata is utilized as a traditional Chinese medicine for the treatment of inflamed wounds, arthritis, rheumatism arthralgia, and trichomonas vaginalis. Moreover, M. cordata has been extensively used in stockbreeding and agriculture [5].
Previous chemical and pharmacological research has revealed that alkaloids are considered to be responsible for the major pharmacological effects of M. cordata, such as antimicrobial, antifungal, anti-inflammatory, anticancer, adrenolytic, sympatholytic, and local anesthetic activities [6-11]. The main alkaloids in the plant consist of benzophenanthridines, protoberberines, protopines and other types [11]. M. cordata had attracted much attention in recent decades, on account of the intriguing structures and significant biological activities. Our group has been long focusing on the discovery of structurally unique natural products with potent bioactivity from traditional Chinese medicines [12-15]. Moreover, in the course of our ongoing search for new and bioactive alkaloids, the continued investigation of the CH2Cl2 fraction from M. cordata [16] yielded a novel alkaloid, macleayine (1) (Fig. 1), with a unique carbon skeleton featuring a spiro [furanone-piperidinedione] system. To the best of our knowledge, 1 is the first aza-spiroketal compound comprising a spiro [benzofuranone and benzopiperidinedione] framework in natural products. Herein, details of the isolation, structural elucidation, and activity evaluation of 1 are described. In addition, a plausible biogenetic pathway of 1 is proposed.

|
Download:
|
| Figure 1. Structure of macleayine (1). | |
2. Experimental 2.1. General experimental procedures
UV spectrum was recorded using a Shimadzu UV-2201 spectrometer. 1D and 2D NMR spectra were acquired with Bruker AV-600 NMR spectrometers using solvent signals (DMSO-d6: δH 2.50/δC 39.5), with tetramethylsilane (TMS) as an internal standard. HR-ESIMS spectrum was obtained using a Bruker micrOTOF-Q mass spectrometer. Column chromatography (CC) was performed with silica (100-200 and 200-300 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). ODS (50 μm, YMC Co., Ltd., Kyoto, Japan) and Sephadex LH-20 (GE Healthcare, Sweden). TLC analysis was carried out with glass plate precoated silica gel (GF254, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China).
2.2. Plant materialThe plant material was purchased from Anguo Medicines Ltd. (Hebei), China, in November 2013 and was identified as the aerial parts of Macleaya cordata (Willd.) R. Br. by Prof. Jin-Cai Lu (School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, China). The voucher sample (BLH-20131108) was deposited in the Department of Natural Products Chemistry, Shenyang Pharmaceutical University, Shenyang, China.
2.3. Extraction and isolationThe air-dried aerial parts of M. cordata (40.0 kg) were extracted with 95% EtOH (400 L) under reflux for 2 times, followed by 75% EtOH (1× 400 L). The combined extract was concentrated in vacuo to yield a residue, which was suspended in H2O, successively partitioned with CH2Cl2 and n-BuOH, to afford the CH2Cl2, n-BuOH, and aqueous extracts. Part of the CH2Cl2 soluble portion (500 g) was fractionated using silica gel column chromatography (CC) eluted with petroleum ether (60-90 ℃)-acetone (100:5, 100:10, 100:20, 100:50, 100:100 and 0:100, v/v) to yield six fractions (Fr. A-Fr. F). Fr. D (100:50, v/v) was further subjected to ODS CC with stepwise elution using MeOH-H2O (50:50, 60:40, 65:35, 70:30, 80:20, 90:10 and 100:0, v/v) as the mobile phase to give seven subfractions (Fr. D1-Fr. D7). Fr. D1 (50:50, v/v) was further separated by Sephadex LH-20 with CH2Cl2: MeOH (1:1, v/v) to yield compound 1 (2 mg).
Macleayine (1): White solid; UV (MeOH)λmax: 227, 302, 349 nm; ECD (MeOH): 274 nm (△ε + 2.38) and 231 nm (△ε -17.79); 1H NMR (600 MHz, DMSO-d6) and 13C NMR (150 MHz, DMSO-d6) data, see Table 1; HRESIMS m/z 382.0556 [M+H]+ (calcd. 382.0557).
|
|
Table 1 1H NMR and 13C NMR data for 1 in DMSO-d6.a |
2.4. Cytotoxicity test for macleayine
The cytotoxicity of macleayine (1) against human cancer HL-60, HeLa, MCF-7, and BGC-823 cell lines were tested by trypan blue method and MTT method [17, 18], with 5-fluorouracil as a positive control. Unfortunately, no significant activity was detected.
2.5. Docking studiesVirtual molecular docking study was performed to investigate interaction of macleayine (1) with protein tyrosine phosphatase 1B (PTP1B), sequentially to predict the activity of ligand. The target of 1 was searched by the target protein online search tools-pharmmapper server, with z'-score >3 and number of feature >5 to restrict. The target proteins in the Protein Data Bank database (PDB) were selected with resolutio >2 Å, and ultimately the five eligible receptor proteins with 1 docking were determined. The results (Fig. S4 in Supporting information) of virtual screening showed 1 binding better with protein 1NO6 (PDB ID), which means this compound is a potent, competitive inhibitor of PTP1B. Moreover, PTP1B is an enzyme that downregulates the insulin receptor [19], so the compound has the potent effect of treating type Ⅱ diabetes.
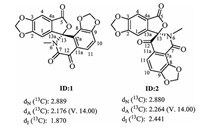
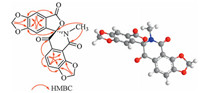
3. Results and discussionCompound 1, obtained as a white cluster crystal (in CH2Cl2: MeOH=1:1), was determined to have the molecular formula C19H11NO8 on the basis of positive HR-ESIMS (m/z 382.0556 [M+H]+ Studies, calcd. 382.0557), with 15 degrees of unsaturation. The UV spectrum showed the maximum absorptions at 227, 302, and 349 nm. Analysis of the 1H NMR data of 1 revealed the presence of two uncoupled aromatic protons [δH 7.43 (s, 1H, H-4); 7.35 (s, 1H, H-1)], two ortho-coupled aromatic protons [δH 7.64 (d, 1H, J=8.1 Hz); 7.37 (d, 1H, J=8.1 Hz)], two methylenedioxyls [δH 6.41, 6.40, 6.28, 6.26 (each s, 1H)], and one N-methyl [δH 2.70 (s, 3H)]. The 13C NMR (Table 1) and HSQC spectra showed characteristic signals of three carbonyls (including one conjugated ketone carbonyl at δC 183.0, one ester carbonyl at δC 166.7, and one amide carbonyl at δC 159.0), twelve aromatic carbons (including eight quaternary carbons and four protonated aromatic carbons), two methylenedioxyls at δC 103.6 and 104.2, a sp3 quaternary atom at δC 94.2, and one N-methyl at δC 28.8. Comparison of the above data with those of the previously reported alkaloids from this plant prompted us to consider compound 1 comprising A, E, D, and F moieties (Fig. 1) of benzophenanthridine alkaloids [20]. The HMBC correlations from H-1 to C-2, C-3, C-4a, and C-13, from H-4 to C-2, C-3, C-5, and C-13a, and from methylenedioxyl protons to C-2 and C-3 indicated the existence of rings A, B, and E (Fig. 2). The correlations of H-10 with C-8, C-9 and C-11a, H-11 with C-7a, C-9, C-10 and C-12, and of N-methyl protons with C-7 and C-13 revealed existence of rings C, D, and F. Furthermore, the observation of HMBC correlations between H-1/N-methyl protons and C-13 indicated a furanone ring B was connective to piperidinedione ring C via a spiro atom (C-13) (Fig. 3). The structure could not be unambiguously established due to the paucity of correlations between ring B and C. Therefore, the spectroscopic data collected for 1 were analyzed using the ACD/ Structure Elucidator (Struc-Eluc; ACD/Labs, Toronto, ON, Canada) [21]. Struc-Eluc suggested two feasible structures ID:1 and ID:2, shown in Fig. 2. Subsequently, 13C NMR chemical shift calculations [22] were carried out by quantum chemistry methods. In the second case (ID:2), all of the maximum of relative errors of chemical shift were below 8.0 ppm. However, in the first case (ID:1), the maximum errors at C-7a and C-12 were 10.1 and 10.9 ppm, respectively (see ESI). Thus, the structure of ID:2 is more acceptable for 1. Therefore, the planar structure of 1 was determined, as shown in Fig. 1.
|
Download:
|
| Figure 2. The suggested feasible structures by structure elucidator. | |
|
Download:
|
| Figure 3. HMBC correlations and 3D structure of 1. | |
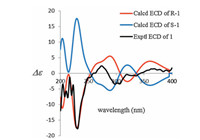
In order to establish the absolute configuration of compound 1, extensive efforts to obtain an applicable single crystal were undertaken, but unfortunately failed. The electronic circular dichroism (ECD) spectrum of 1 was measured, and then the experimental ECD spectrum was compared with the calculated ECD data by the time dependent density functional theory (TD-DFT) method at B3LYP/6-31G(d) level [23]. The ECD spectrum of 1 showed the Cotton effects at 274 nm (△ε + 2.38) and 231 nm (△ε -17.79), which resulted from the exciton coupling of two different chromophores (benzofuranone and benzopiperidinedione). The experimental ECD spectrum (measured in methanol) of 1 matched well with the calculated spectrum of R-1 in the region of 200-400 nm (Fig. 4, for details see Supporting information), unambiguously certifying that it was the most reasonable absolute configuration.
|
Download:
|
| Figure 4. Experimental and calculated ECD spectra of 1. | |
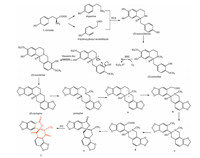
A plausible biogenetic pathway of 1 was postulated (Scheme 1). The biosynthetic precursor of macleayine (1) could be admitted to protopine, a major alkaloid of M. cordata, which might be generated from two L-tyrosine-derived dopamine and 4-hydroxyphenyl acetaldehyde in the presence of norcoclaurine synthase (NCS) to yield (S)-norcoclaurine [24]. Conversion from (S)-norcoclaurine to (S)-scoulerine involved hydroxylation, O-methylation and N-methylation, followed by further oxidation and Mannich-like reaction. The (S)-scoulerine then was catalysed and yielded (S)-stylopine with the help of two P450-dependent enzymes, cheilanthifoline synthase (CFS) and stylopine synthase (STS) [3, 25], and further converted to protopine via N-methylation and oxidation cleavage. Cleavage of the heterocyclic ring systems adjacent to the nitrogen atom produced b, and then gave d by decarboxylation and oxidation. Compound 1 could be finally produced from d by self-lactonization and oxidation [26].
|
Download:
|
| Scheme. 1. A plausible biogenetic pathway of macleayine (1). | |
4. Conclusion
In summary, macleayine (1) was the first example of natural alkaloid with the spiro [benzofuranone-benzopiperidinedione] skeleton. The structure including absolute configuration was determined on the basis of spectroscopic analyses along with calculated ECD method. Some natural analogues of 1 are medicinally important, such as, spirobenzylisoquinoline-type alkaloids showed antiviral activity against Hepatitis B virus [27], and a phthalide type alkaloid bicuculline was widely applied as pharmacological probes for convulsants acting at GABA neuroreceptors [28]. Therefore, compound 1 is worthy of further investigation, including its biological activities and synthesis. The result of virtual molecular docking predicted the compound can enhance the effects of insulin, and may treat type Ⅱ diabetes. Unfortunately, no sample was available for the further bioassay, but this will attract extensive concern and intense interest from researchers.
AcknowledgmentsThe work was financially supported by the National Natural Science Foundation of China (No. 81172958), the Basic Research Subject of Key Laboratory Supported by Educational Commission of Liaoning Province of China (No. LZ2014044). We gratefully acknowledge Mr. Yi Sha and Mrs. Wen Li, Department of Analytical Testing Center, Shenyang Pharmaceutical University, for measurement of the NMR data, and acknowledge Professor Hua-Jie Zhu and Mr. Fei Cao, Hebei University, for 13C NMR calculation.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.017.
| [1] | G.P. Pi, P. Ren, J.M. Yu, Separation of sanguinarine and chelerythrine in Macleaya cordata (Willd) R. Br. based on methyl acrylate-co-divinylbenzene macroporous adsorbents. J. Chromatogr. A 1192 (2008) 17–24. DOI:10.1016/j.chroma.2008.03.039 |
| [2] | Z.X. Qing, P. Cheng, X.B. Liu, Structural speculation and identification of alkaloids in Macleaya cordata fruits by high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening procedure. Rapid Commun. Mass Spectrom. 28 (2014) 1033–1044. DOI:10.1002/rcm.6874 |
| [3] | J.G. Zeng, Y.S. Liu, W. Liu, Integration of transcriptome, proteome and metabolism data reveals the alkaloids biosynthesis in Macleaya cordata and Macleaya microcarpa. PLoS ONE 8 (2013) e53409. DOI:10.1371/journal.pone.0053409 |
| [4] | M. Liu, Y.L. Lin, X.R. Chen, In vitro assessment of Macleaya cordata crude extract bioactivity and anticancer properties in normal and cancerous human lung cells. Exp. Toxicol. Pathol. 65 (2013) 775–787. DOI:10.1016/j.etp.2012.11.004 |
| [5] | P. Kosina, J. Gregorova, J. Gruz, Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia 81 (2010) 1006–1012. DOI:10.1016/j.fitote.2010.06.020 |
| [6] | A. Zdarilova, E. Vrublova, J. Vostalova, Natural feed additive of Macleaya cordata: safety assessment in rats a 90-day feeding experiment. Food Chem. Toxicol. 46 (2008) 3721–3726. DOI:10.1016/j.fct.2008.09.054 |
| [7] | T.K. Beuria, M.K. Santra, D. Panda, Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry 44 (2005) 16584–16593. DOI:10.1021/bi050767+ |
| [8] | X.F. Niu, T. Fan, W.F. Li, The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur. J. Pharmacol. 689 (2012) 262–269. DOI:10.1016/j.ejphar.2012.05.039 |
| [9] | Y.Z. Chen, G.Z. Liu, Y. Shen, Analysis of alkaloids in Macleaya cordata (Willd.) R. Br. using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatogr. A 1216 (2009) 2104–2110. DOI:10.1016/j.chroma.2008.08.066 |
| [10] | J. Ulrichová, Z. Dvořák, J. Vičar, Cytotoxicity of natural compounds in hepatocyte cell culture models: the case of quaternary benzo. Toxicol. Lett. 125 (2001) 125–132. DOI:10.1016/S0378-4274(01)00430-1 |
| [11] | X.L. Yu, X.L. Gao, Z.X. Zhu, Alkaloids from the tribe Bocconieae (Papaveraceae): a chemical and biological review. Molecules 19 (2014) 13042–13060. DOI:10.3390/molecules190913042 |
| [12] | S.L. Niu, Z.L. Li, F. Ji, Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry 77 (2012) 280–286. DOI:10.1016/j.phytochem.2012.01.010 |
| [13] | K.B. Wang, Y.T. Di, Y. Bao, Peganumine A, a β-carboline dimer with a new octacyclic scaffold from Peganum harmala. Org. Lett. 16 (2014) 4028–4031. DOI:10.1021/ol501856v |
| [14] | K.B. Wang, C.M. Yuan, C.M. Xue, Pegaharmalines A and B, two novel β-carboline alkaloids with unprecedented carbon skeletons from Peganum harmala. RSC Adv. 4 (2014) 53725–53729. DOI:10.1039/C4RA07985J |
| [15] | N. Zhao, Z.L. Li, D.H. Li, Quinolone and indole alkaloids from the fruits of Euodia rutaecarpa and their cytotoxicity against two human cancer cell lines. Phytochemistry 109 (2015) 133–139. DOI:10.1016/j.phytochem.2014.10.020 |
| [16] | C.M. Sai, D.H. Li, C.M. Xue, Two pairs of enantiomeric alkaloid dimers from Macleaya cordata. Org. Lett. 17 (2015) 4102–4105. DOI:10.1021/acs.orglett.5b02044 |
| [17] | F. Wang, H.M. Hua, Y.H. Pei, Triterpenoids from the resin of Styrax tonkinensis and their antiproliferative and differentiation effects in human leukemia HL-60 cells. J. Nat. Prod. 69 (2006) 807–810. DOI:10.1021/np050371z |
| [18] | J. Hu, X.D. Shi, J.G. Chen, Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 148 (2014) 437–444. DOI:10.1016/j.foodchem.2012.12.058 |
| [19] | B.G. Szczepankiewicz, G. Liu, P.J. Hajduk, Discovery of a potent, selective protein tyrosine phosphatase 1B inhibitor using a linked-fragment strategy. J. Am. Chem. Soc. 125 (2003) 4087–4096. DOI:10.1021/ja0296733 |
| [20] | A.J. Deng, H.L. Qin, Cytotoxic dihydrobenzophenanthridine alkaloids from the roots of Macleaya microcarpa. Phytochemistry 71 (2010) 816–822. DOI:10.1016/j.phytochem.2010.02.007 |
| [21] | C.B. Naman, J. Li, A. Moser, Computer-assisted structure elucidation of black chokeberry (Aronia melanocarpa) fruit juice isolates with a new fused pentacyclic flavonoid skeleton. Org. Lett. 17 (2015) 2988–2991. DOI:10.1021/acs.orglett.5b01284 |
| [22] | F. Cao, Q. Yang, C.L. Shao, Bioactive 7-oxabicyclic[6.3.0] lactam and 12-membered macrolides from a gorgonian-derived Cladosporium sp. Fungus. Mar. Drugs 13 (2015) 4171–4178. DOI:10.3390/md13074171 |
| [23] | J.L. Zhang, H.Y. Tian, N.H. Chen, Caesalpinimin a, a novel rearranged furanoditerpene with an unprecedented carbon skeleton from the seeds of Caesalpinia minax Hance. RSC Adv. 4 (2014) 7440–7443. DOI:10.1039/c3ra46502k |
| [24] | J.M. Finefield, D.H. Sherman, M. Kreitman, Enantiomeric natural products: occurrence and biogenesis. Angew. Chem. Int. Ed. 51 (2012) 4802–4836. DOI:10.1002/anie.v51.20 |
| [25] | J. Ziegler, P.J. Facchini, Alkaloid biosynthesis: metabolism and trafficking. Annu. Rev. Plant Biol. 59 (2008) 735–769. DOI:10.1146/annurev.arplant.59.032607.092730 |
| [26] | P.M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, 2nd ed., John Wiley & Sons Ltd.: Chichester, England, 2003 : pp. 339 -340. |
| [27] | M. Aljofan, H.J. Netter, A.N. Aljarbou, Anti-hepatitis B activity of isoquinoline alkaloids of plant origin. Arch. Virol. 159 (2014) 1119–1128. DOI:10.1007/s00705-013-1937-7 |
| [28] | M. Rewal, M.E. Jung, J.W. Simpkins, Role of the GABA-A system in estrogeninduced protection against brain lipid peroxidation in ethanol-withdrawn rats. Alcohol. Clin. Exp. Res. 28 (2004) 1907–1915. DOI:10.1097/01.ALC.0000148100.78628.E7 |
 2016, Vol. 27
2016, Vol. 27 



