b Key Laboratory for Green Pharmaceutical Technologies and Related Equipment of Ministry of Education, College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310014, China
Over the past decades, green chemistry has evoked increasing interest in new, environmentally benign procedures such as multicomponent reactions, solvent-free syntheses, and reusable catalysts to save resources and energy [1]. Among these, multicomponent reactions (MCRs) are a very useful tool in synthetic organic chemistry as well as in drug discovery programs. With the developing awareness of environmentally benign chemical syntheses among the scientific community, designing chemical reactions without hazardous chemical ingredients to reduce or eliminate toxic waste and byproducts is the utmost priority for synthetic chemists. As organic solvents (THF, DMSO, DMF, CHCl3, CCl4, etc.) are considered to be the highest contributors to environmental pollution, synthetic utility is further made more attractive when environmentally-friendly solvents such as ethanol or water are used. The discovery of novel synthetic methodologies to prepare compound libraries using MCRs without the use of hazardous solvents is a significant and pivotal focal point in industry and academia [2].
The dihydropyrano[2, 3-c]pyrazoles play an essential role as versatile synthetic building blocks and pharmacophores. Many of those compounds show different pharmacological effects such as antimicrobial [3], insecticidal [4], anti-inflammatory [5] and molluscicidal [6] activities. Furthermore, dihydropyrano[2, 3-c]pyrazoles are reported as pharmaceutical ingredients, Chk1 inhibitors [7], and biodegradable agrochemicals [8].
In general, pyrano[2, 3-c]pyrazoles have been synthesized via two-component reaction [9] involving pyran derivatives and hydrazine hydrate; three-component condensation [10] between N-methylpiperidone, pyrazolin-5-one and malononitrile in absolute ethanol; and more importantly four-component reactions of aldehyde, ethyl acetoacetate, hydrazine and malononitrile [11]. However, most of the protocols used nitrogenous based unrecoverable homogeneous catalysts like triethylamine [11a], piperidine [11b], L-proline [11c, d], per-6-amino-β-cyclodextrin [11e], hexadecyl dimethyl benzyl ammonium chloride [11f], basic ionic liquids [11g, h], disulfonic acid imidazolium chloroaluminate [11i], and meglumine [11j]. Several methods involving heterogeneous catalysts, such as amberlyst A21 [11k], γ-alumina [11l], and SnO2 QDs [11m], have been reported. To the best of our knowledge, there are few methods available for the synthesis of highly functionalized dihydropyrano[2, 3-c]pyrazoles frameworks in the presence of a Lewis acid. Thus, the development of a general MCR protocol using green Lewis acid catalyst leading to the pyrano[2, 3-c]pyrazoles derivatives is highly desirable.
Organocatalysis is becoming an interesting area as it avoids the use of expensive and toxic metals. Ammonium triflate is a novel organocatalyst which has been applied in a variety of reactions and displayed great catalytic activity and efficiency [12]. Furthermore, ammonium triflate has many advantages, including easy separation, good reusability and environmental acceptability compared to traditional Lewis acid catalysts. In connection with our continuing studies on the development of one-pot multicomponent reactions catalyzed by ammonium triflates, we synthesized benzoxanthenes catalyzed by proline triflate (ProT) [12a] and 1, 4-dihydropyridines catalyzed by diphenylammonium triflate (DPAT) [12b]. We also investigated some other reactions catalyzed by DPAT [12c] or ProT [12d]. Herein, we report an efficient and environmentally friendly method for the synthesis of dihydropyrano[2, 3-c]pyrazoles catalyzed by MorT.
2. ExperimentalAnalytical grade solvents and commercially available reagents were used without further purification. Melting points were determined on a Büchi B-540 capillary melting point apparatus and uncorrected. All 1H NMR and 13C NMR spectra were recorded on a VARAIN-400 using DMSO-d6 as the solvent with tetramethylsilane (TMS) as an internal standard. Chemical shifts are given inδrelative to TMS; the coupling constants J are given in Hz. Mass spectra were measured with a Thermo Finnigan LC Advantage (Agilent 1100). High resolution mass spectrometry (HRMS) was performed on an Agilent 6210 TOF LC/MS using ESI or EI (electrospray ionization) techniques.
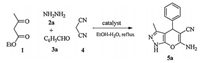
General procedure for synthesis of dihydropyrano[2, 3-c]pyrazoles (5) (Scheme 1): To a pre-stirred mixture of ethyl acetoacetate (1) (0.26 mL, 2.0 mmol), hydrazine hydrate (2)(0.13 mL, 2.5 mmol) in EtOH/H2O (v/v=9:1, 6 mL) was added aldehydes (3) (2.0 mmol) and malononitrile (4) (0.13 g, 2.0 mmol) followed by MorT (10 mol%). The resulting mixture was stirred under reflux. After completion of the reaction (monitored by TLC, n-hexane/ethyl acetate=3:1), the precipitated product was filtered and washed with aqueous ethanol (10 mL). The crude residue was crystallized from ethanol/water (v/v=9.5:0.5). 1H NMR and 13C NMR spectra for all compounds are available in Supporting information.
|
Download:
|
| Scheme. 1. General procedure for synthesis of dihydropyrano[2, 3-c]pyrazoles. | |
Typical spectral data of some compounds are listed below, others are deposited in Supporting information.
6-Amino-3-methyl-4-phenyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5a): Mp: 247-248 °C. 1H NMR (DMSO-d6, 400 MHz):δ12.07 (s, 1H), 7.32-7.28 (m, 2H), 7.22-7.19 (m, 1H), 7.15 (d, 2H, J=7.2 Hz), 6.86 (s, 2H), 4.58 (s, 1H), 1.77 (s, 3H); 13C NMR (DMSO-d6, 100 MHz):δ160.5, 154.4, 144.1, 135.2, 128.1, 128.1, 127.2, 127.2, 126.4, 120.5, 97.4, 57.2, 36.2, 9.7; MS (ESI): m/z 251.3 [M-H]-.
6-Amino-4-(4-fluorophenyl)-3-methyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5b): Mp: 223-224 °C. 1H NMR (DMSO-d6, 400 MHz):δ12.09 (s, 1H), 7.21-7.17 (m, 2H), 7.12 (t, 2H, J=8.8 Hz), 6.89 (s, 2H), 4.62 (s, 1H), 1.78 (s, 3H); 13C NMR (DMSO-d6, 100 MHz):δ161.8, 160.5, 159.4, 154.4, 140.4, 135.3, 129.0, 120.4, 115.0, 114.8, 97.3, 57.1, 35.4, 9.7; MS (ESI): m/z 269.3 [M-H]-.
6-Amino-3-methyl-4-(p-tolyl)-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5j): Mp: 208-209 °C. 1H NMR (DMSO-d6, 400 MHz):δ12.05 (s, 1H), 7.10 (d, 2H, J=8.0 Hz), 7.03 (d, 2H, J=8.0 Hz), 6.82 (s, 2H), 4.53 (s, 1H), 2.26 (s, 1H), 1.78 (s, 3H); 13C NMR (DMSO-d6, 100 MHz):δ160.4, 154.5, 141.2, 135.4, 135.3, 128.7, 128.7, 127.1, 127.1, 120.6, 97.6, 57.4, 35.9, 20.7, 9.8; MS (ESI): m/z 265.3 [M-H]-.
6-Amino-3-methyl-4-(4-nitrophenyl)-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5n): Mp: 250-251 °C. 1H NMR (DMSO-d6, 400 MHz):δ12.17 (s, 1H), 8.19 (d, 2H, J=8.8 Hz), 7.45 (d, 2H, J=8.8 Hz), 7.04 (s, 2H), 4.82 (s, 1H), 1.80 (s, 3H); 13C NMR (DMSO-d6, 100 MHz):δ160.8, 154.4, 151.7, 146.1, 135.6, 128.6, 128.6, 123.6, 123.6, 120.2, 96.4, 55.9, 35.9, 9.8; MS (ESI): m/z 296.3 [M-H]-.
6-Amino-4-(2, 4-dichlorophenyl)-3-methyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5r): Mp: 220-221 °C. 1H NMR (DMSO-d6, 400 MHz):δ12.14 (s, 1H), 7.57 (d, 1H, J=2.0 Hz), 7.39 (dd, 1H, J=8.4 Hz), 7.20 (d, 1H, J=8.0 Hz), 7.00 (s, 2H), 5.05 (s, 1H), 1.78 (s, 3H); 13C NMR (DMSO-d6, 100 MHz):δ161.0, 154.6, 139.8, 135.2, 132.6, 131.9, 131.9, 128.6, 127.8, 120.0, 96.2, 55.3, 33.1, 9.4; MS (ESI): m/z 319.2 [M-H]-.
6-Amino-4-(3, 4-dimethylphenyl)-3-methyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5s): Mp: 201-202 °C. 1H NMR (DMSO-d6, 400 MHz):δ12.02 (s, 1H), 7.04 (d, 1H, J=7.6 Hz), 6.88-6.85 (m, 2H), 6.80 (s, 2H), 4.48 (s, 1H), 2.17 (s, 6H), 1.78 (s, 3H); 13C NMR (DMSO-d6, 100 MHz):δ160.4, 154.4, 141.6, 135.8, 135.3, 134.2, 129.2, 128.2, 124.7, 120.6, 97.6, 57.5, 35.9, 19.5, 19.0, 9.8; MS (ESI): m/z 303.5 [M+Na]+. HRMS (ESI+, m/z): [M+H]+, calcd. for C16H17N4O: 281.1397, found: 281.1399.
6-Amino-3-methyl-1, 4-diphenyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5aa): Mp: 168-169 °C. 1H NMR (DMSO-d6, 400 MHz):δ7.78(d, 2H, J=7.6 Hz), 7.50-7.46(m, 2H), 7.34-7.26(m, 3H), 7.26-7.24 (m, 3H), 7.22 (s, 2H), 4.67 (s, 1H), 1.78 (s, 3H); 13C NMR(DMSO-d6, 100 MHz): δ159.1, 144.9, 143.6, 143.3, 137.3, 129.0, 129.0, 128.2, 128.2, 127.5, 127.5, 126.7, 125.8, 119.7, 119.7, 119.7, 98.4, 58.2, 36.7, 12.6; MS (ESI): m/z 329.4 [M+H]+. HRMS (ESI+, m/z): [M+H]+, calcd. for C20H17N4O: 329.1397, found: 329.1406.
6-Amino-4-(4-isopropylphenyl)-3-methyl-1-phenyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (5af): Mp: 149-151 °C. 1H NMR (DMSO-d6, 400 MHz):δ7.77 (d, 2H, J=7.6 Hz), 7.49-7.45 (m, 2H), 7.32-7.28 (m, 1H), 7.21 (m, 2H), 7.19 (s, 2H), 7.15-7.13 (m, 2H), 4.63 (s, 1H), 2.90-2.83 (m, 1H), 1.79 (s, 3H), 1.19 (d, 6H, J=7.2 Hz); 13C NMR (DMSO-d6, 100 MHz):δ159.1, 146.6, 144.9, 143.5, 140.7, 137.3, 129.0, 129.0, 127.3, 127.3, 126.1, 126.1, 125.8, 119.8, 119.7, 119.7, 98.6, 58.2, 36.3, 33.0, 23.8, 23.8, 12.6; MS (ESI):m/z 371.5 [M+H]+. HRMS (ESI+, m/z): [M+H]+, calcd. for C23H23N4O:371.1866, found: 371.1866.
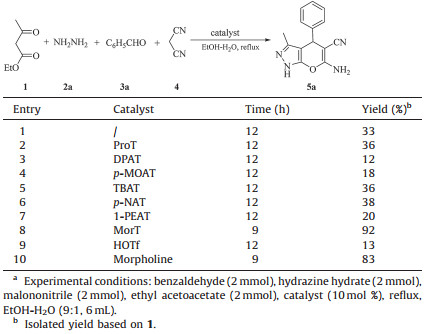
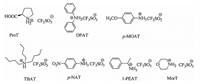
3. Results and discussionWe started our investigation by choosing different ammonium triflates (Fig. 1) for the optimization of reaction conditions catalyzing this four-component condensation using EtOH/H2O as solvent (Table 1).
|
Download:
|
| Figure 1. Different kinds of ammonium triflates. | |
|
|
Table 1 Influence of different catalysts.a |
When the reaction was conducted without catalyst, only a low yield of product was obtained even after 12 h (Table 1, entry 1). This result suggested that catalyst played a critical role in this reaction. A number of ammonium triflate catalysts were examined to promote this reaction under reflux (Table 1, entries 2-8). MorT proved to be the most efficient one that gave the highest yield (92%) within 9 h (Table 1, entry 8). Morphine and HOTf were also examined to promote this transformation, as they can react with each other to afford MorT (Table 1, entries 9 and 10). It seemed that the catalytic activity of MorT was much better than HOTf but close to morphine. There are many papers related to the synthesis of dihydropyrano[2, 3-c]pyrazoles catalyzed by Lewis acid. Here, MorT was introduced as the Lewis acid catalyst leading to the pyrano[2, 3-c]pyrazoles.
With regard to the choice of catalyst, we carried out the above reaction in various solvents. As shown in Table 2, when the reaction was performed under solvent-free conditions, low product yield was obtained (Table 2, entry 1). To find the best solvent for this transformation, the present four-component reaction was screened in H2O, THF, DMSO, DMF, MeOH, EtOH, iPrOH and ethanol-water mixture. Among all these solvents, ethanol-water (9:1) was found to be the best one and afforded the highest yield (Table 2, entry 11). Therefore, ethanol-water was selected as the solvent system for the subsequent reaction.
|
|
Table 2 Optimization of reaction conditions. a |
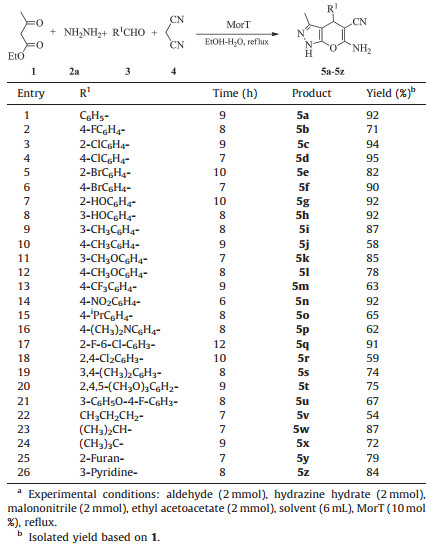
Encouraged by the efficiency of the reaction protocol described above, the scope and specificity of this protocol were further investigated. A library of dihydropyrano[2, 3-c]pyrazoles were constructed under the optimized reaction conditions (Table 3). A broad range of structurally diverse aldehydes were treated with hydrazine hydrate, malononitrile, and ethyl acetoacetate, and the results are depicted in Table 3. All reactions proceeded efficiently, and the desired products were obtained in moderate to excellent yields.
|
|
Table 3 Synthesis of dihydropyrano[2, 3-c]pyrazole 5 from carbonyl compounds.a |
Irrespective of the presence of electron withdrawing or donating substituents in the ortho, meta, or para positions on the aromatic ring of aldehydes, the reactions proceeded smoothly to furnish the desired products in high yields (5a-5z). The reaction was relatively sensitive to the steric environment of the aromatic aldehydes, and longer reaction time was required for benzaldehyde containing substituents at 2-position (Table 3, entries 3, 5, 7, 17 and 18). A relatively low yield was observed when the aromatic aldehydes were occupied by two or more substituents (Table 3, entries 18-21). The reaction worked well for the aliphatic aldehydes under similar reaction conditions without the unwanted byproducts via side reactions such as aldo condensation and the Cannizzaro reaction (Table 3, entries 21-24). Heteroaromatic aldehydes, such as furan-2-carbaldehyde and pyridine-3-carboxaldehyde readily participated in this transformation, affording the pyranopyrazoles in high yields (Table 3, entries 25 and 26). In general, all the reactions listed in Table 3 were moderate to highyielding (54%-95%) and in non-hazardous solvents (EtOH-H2O) and the products could be recrystallized from ethanol to avoid column chromatography purification.
Additionally, the reaction went well when hydrazine hydrate was replaced with phenylhydrazine. We observed that it was a little sensitive to the steric environment of the hydrazine as a slightly longer reaction time was required for the reaction compared with Table 3 (Table 4, entries 1-6).
|
|
Table 4 Synthesis of dihydropyrano[2, 3-c]pyrazole 5 from phenylhydrazine and carbonyl compounds.aa |
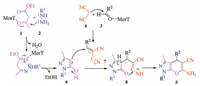
The formation of product 5 is proposed to involve the following tandem reactions (Scheme 2): Firstly, pyrazolone 6 was formed by the reaction between 1 and 2. The Knoevenagal condensation between 3 and 4 was carried out in the presence of MorT. Then, after Michael addition of 6 and 7, followed by cyclization and tautomerization, the title product 5 was formed.
|
Download:
|
| Scheme. 2. Proposed mechanism for the formation of pyrano[2, 3-c]pyrazoles. | |
4. Conclusion
In summary, we have developed an efficient method for the synthesis of a diverse range of dihydropyrano[2, 3-c]pyrazoles using Lewis acid MorT as an eco-friendly catalyst. No chromatography, no hazardous organic solvents, and moderate to excellent yield of the products are the major achievements of this reaction protocol, which has potential to be extremely useful for synthetic applications. This method using Lewis acid is a useful supplement to the reported methods.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.05.010.
| [1] |
(a) C. Allais, J.M. Grassot, J. Rodriguez, T. Constantieux, Metal-free multicomponent syntheses of pyridines, Chem. Rev. 114(2014) 10829-10868; (b) K. Tanaka, F. Toda, Solvent-free organic synthesis, Chem. Rev. 100(2000) 1025-1074; (c) A. Roucoux, J. Schulz, H. Patin, Reduced transition metal colloids: a novel family of reusable catalysts, Chem. Rev. 102(2002) 3757-3778. |
| [2] |
(a) L.A. Thompson, Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis, Curr. Opin. Chem. Biol. 4(2000) 324-337; (b) A. Nefzi, J.M. Ostresh, R.A. Houghten, The current status of heterocyclic combinatorial libraries, Chem. Rev. 97(1997) 449-472. |
| [3] | S.P. Prajapati, D.P. Patel, P.S. Patel, Synthesis, characterization and antimicrobial activity of 6-amino-4-(substitutedphenyl)-1-(2, 4-dinitrophenyl)-3-methyl-1, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile derivatives. J Chem. Pharm. Res. 4 (2012) 2652–2655. |
| [4] | S.A. Chaudhari, S.R. Patil, V.M. Patil, Synthesis of pyrano[2, 3-d]pyridine, pyrazolo[3, 4-b]pyridine derivatives by microwave irradiation and study of their insecticidal activity. J. Chem. Pharm. Res. 7 (2015) 476–482. |
| [5] | M.E.A. Zaki, H.A. Soliman, O.A. Hiekal, A.E. Rashad, Pyrazolopyranopyrimidines as a class of anti-inflammatory agents. Z. Naturforsch. C 61 (2006) 1–5. |
| [6] |
(a) F.M. Abdelrazek, P. Metz, N.H. Metwally, et al., Synthesis and molluscicidal activity of new cinnoline and pyrano[2, 3-c] pyrazole derivatives, Arch. Pharm. Chem. Life Sci. 339(2006) 456-460; (b) F.M. Abdelrazek, P. Metz, O. Kataeva, et al., Synthesis and molluscicidal activity of new chromene and pyrano[2, 3-c]pyrazole derivatives, Arch. Pharm. Chem. Life Sci. 340(2007) 543-548. |
| [7] |
(a) N. Foloppe, L.M. Fisher, R. Howes, et al., Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening, Bioorg. Med. Chem. 14(2006) 4792-4802; (b) A. Kimata, H. Nakagawa, R. Ohyama, T. Fukuuchi, et al., New series of antiprion compounds: pyrazolone derivatives have the potent activity of inhibiting protease-resistant prion protein accumulation, J. Med. Chem. 50(2007) 5053-5056. |
| [8] |
(a) A.M.G. James, J.S. Peter, Heterocyclic tautomerism, part 12. The structure of the product of reaction between 3-methyl-1-phenyl-2-pyrazolin-5-one and tetracyanoethylene, Arkivoc 2(2001) 32-36; (b) L.A.Rodinovskaya, A.V.Gromova, A.M.Shestopalov, etal., Synthesisof6-amino-4-aryl-5-cyano-3-(3-cyanopyridin-2-ylthiomethyl)-2, 4-dihydropyrano[2, 3-c]pyrazoles and their hydrogenated analogs. Molecular structure of 6-amino-5-cyano-3-(3-cyano-4, 6-dimethylpyridin 2-ylthiomethyl)-4-(2-nitrophenyl)-2, 4-dihydropyrano[2, 3-c]pyrazole, Russ, Chem. Bull. Int. Ed. 52(2003) 2207-2213. |
| [9] | Y.Q. Peng, G.H. Song, R.L. Dou, Surface cleaning under combined microwave and ultrasound irradiation: flash synthesis of 4H-pyrano[2, 3-c] pyrazoles in aqueous media. Green Chem. 8 (2006) 573–575. DOI:10.1039/b601209d |
| [10] |
(a) A.M. Shestopalov, Y.M. Emeliyanova, A.A. Shestopalov, et al., Cross-condensation of derivatives of cyanoacetic acid and carbonyl compounds. Part 1: singlestage synthesis of 1’-substituted 6-amino-spiro-4-(piperidine-4)-2H, 4H-pyrano[2, 3-c]pyrazole-5-carbonitriles, Tetrahedron 59(2003) 7491-7496; (b) A.M. Shestopalov, Y.M. Emeliyanova, A.A. Shestopalov, et al., One-step synthesis of substituted 6-amino-5-cyanospiro-4-(piperidine-40)-2H, 4H-dihydropyrazolo[3, 4-b]pyrans, Org. Lett. 4(2002) 423-425. |
| [11] |
(a) Y.M. Litvinov, A.A. Shestopalov, L.A. Rodinovskaya, et al., New convenient four-component synthesis of 6-amino-2, 4-dihydropyrano[2, 3-c]pyrazol-5-carbonitriles and one-pot synthesis of 60-aminospiro[(3H)-indol-3, 40-pyrano[2, 3-c]pyrazol]-(1H)-2-on-50-carbonitriles, J. Comb. Chem. 11(2009) 914-919; (b) G. Vasuki, K. Kumaravel, Rapid four-component reactions in water: synthesis of pyranopyrazoles, Tetrahedron Lett. 49(2008) 5636-5638; (c) J.M. Khurana, B. Nand, S. Kumar, Rapid synthesis of polyfunctionalized pyrano[2, 3-c]pyrazoles via multicomponent condensation in room-temperature ionic liquids, Synth. Commun. 41(2011) 405-410; (d) H. Mecadon, M.R. Rohman, I. Kharbangar, et al., L-Proline as an efficicent catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitriles in water, Tetrahedron Lett. 52(2011) 3228-3231; (e) K. Kanagaraj, K. Pitchumani, Solvent-free multicomponent synthesis of pyranopyrazoles: per-6-amino-b-cyclodextrin as a remarkable catalyst and host, Tetrahedron Lett. 51(2010) 3312-3316; (f) K. Ablajan, L.J. Wang, A. Tuoheti, et al., An efficient four-component, one-pot synthesis of 6-amino-4-aryl-3-methyl-2, 4-dihydropyrano[2, 3-c] pyrazole-5-carbonitriles under phasetransfer catalyst, Lett. Org. Chem. 9(2012) 639-643; (g) J.M. Khurana, A. Chaudhary, Efficient and green synthesis of 4H-pyrans and 4H-pyrano[2, 3-c] pyrazoles catalyzed by task-specific ionic liquid[bmim]OH under solvent-free conditions, Green Chem. Lett. Rev. 5(2012) 633-638; (h) X.J. Li, H.Y. Guo, One-pot synthesis of 1, 4-dihydropyrano[2, 3-c] pyrazoies cataiyzed by basic ionic liquids, Chin. J. Org. Chem. 32(2012) 127-132; (i) A.R. Moosavi-Zare, M.A. Zolfigol, E. Noroozizadeh, et al., Synthesis of 6-amino-4-(4-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1, 4-dihydropyrano[2, 3-c]pyrazoles using disulfonic acid imidazolium chloroaluminate as a dual and heterogeneous catalyst, New J. Chem. 37(2013) 4089-4094; (j) R.Y. Guo, Z.M. An, L.P. Mo, et al., Meglumine promoted one-pot, four-component synthesis of pyranopyrazole derivatives, Tetrahedron 69(2013) 9931-9938; (k) M.Bihani, P.P. Bora, G.Bez, etal., AmberlystA21catalyzedchromatographyfree method for multicomponent synthesis of dihydropyrano[2, 3-c]pyrazoles in ethanol, ACS Sustain. Chem. Eng. 1(2013) 440-447; (l) H. Mecadon, M.R. Rohman, M. Rajbangshi, et al., g-Alumina as a recyclable catalyst for the four-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitriles in aqueous medium, Tetrahedron Lett. 52(2011) 2523-2525; (m) S. Paul, K. Pradhan, S. Ghosh, et al., Uncapped SnO2 quantum dot catalyzed cascade assembling of four components: a rapid and green approach to the pyrano[2, 3-c]pyrazole andspiro-2-oxindole derivatives, Tetrahedron 70(2014) 6088-6099; (n) R.S. Balaskar, S.N. Gavade, M.S. Mane, et al., Greener approach towards the facile synthesis of 1, 4-dihydropyrano[2, 3-c]pyrazol-5-yl cyanide derivatives at room temperature, Chin. Chem. Lett. 21(2010) 1175-1179; (o) N. Iravani, M. Keshavarz, H.A.S. Kish, et al., Tin sulfide nanoparticles supported on activated carbon as an efficient and reusable Lewis acid catalyst for three-component one-pot synthesis of 4H-pyrano[2, 3-c]pyrazole derivatives, Chin. J. Catal. 36(2015) 626-633. |
| [12] |
(a) J.J. Li, L.M. Lu, W.K. Su, A new strategy for the synthesis of benzoxanthenes catalyzed by proline triflate in water, Tetrahedron Lett. 51(2010) 2434-2437; (b) J.J. Li, P. He, C.M. Yu, DPTA-catalyzed one-pot regioselective synthesis of polysubstituted pyridines and 1, 4-dihydropyridines, Tetrahedron 68(2012) 4138-4144; (c) J.J. Li, J. Sun, W.K. Su, Diphenylammonium triflate: a novel and efficient catalyst for synthesis of spiro-heterocyclic compounds, Lett. Org. Chem. 7(2010) 314-318; (d) X.J. Shi, J. Li, W.H. Zhong, et al., Synthesis of 1 H-indazolo[2, 1-b] phthalazinetriones catalysed by proline triflate under solvent-free conditions, J. Chem. Res. 36(2012) 17-20; (e) F. Malamiri, S. Khaksar, Pentafluorophenylammonium triflate (PFPAT): a new organocatalyst for the one-pot three-component synthesis of a-aminophosphonates, J. Chem. Sci. 126(2014) 807-811; (f) B. Karimi, M. Ghoreishi-Nezhad, Highly chemoselective acetalization of carbonyl compounds catalyzed by a novel recyclable ammonium triflate-functionalized silica, J. Mol. Catal. A: Chem. 277(2007) 262-265; (g) A. Del Zotto, W. Baratta, A. Felluga, et al., Addition of secondary amines to activated alkenes promoted by Pd(Ⅱ) complexes: use of ammonium salts as cocatalysts, Inorg. Chim. Acta 358(2005) 2749-2754. |
 2016, Vol. 27
2016, Vol. 27