Vinyl chlorides are one of the most important intermediates in organic synthesis, which have been extensively used as important building blocks for natural and synthetic products with biological activities [1, 2] and also to construct carbon-carbon and carbon-heteroatom bonds through cross-coupling reactions [3-7]. Over the past decades, tremendous efforts have been devoted to the synthesis of vinyl chlorides, commonly from ketones [8-11] and/or alkynes [12-14]. However, stoichiometric phosphorous reagents [8, 9] or acyl chlorides [10, 11] are required to react with ketones to produce vinyl chlorides. Alkynes react with reagents that contain chlorine [13-16], such as acyl chlorides, LiCl, TMSCl, and MgCl2, to form vinyl chlorides in one step or with boron reagents [17, 18] and Al reagent [19] to form vinyl chlorides in two or three steps. In addition, these protocols have disadvantages such as significant waste generation and less efficient multi-step sequences. Based on our continuous interest in the field of alkyne chemistry [20], we recently developed interest in alkyne hydrochlorination, which involves simply adding HCl to the alkyne with 100% atom efficiency and is regarded as a direct and efficient method for producing vinyl chlorides. Very recently, Derien reported the Ru-catalyzed direct and selective alkyne hydrochlorination in excellent yields, which presented a powerful tool for producing vinyl chlorides [21]. However, most reported hydrochlorinations of alkynes have focused on acetylene [22, 23] and hydrochlorination of arylalkynes has received very little attention [21, 24-30]. Several methods require catalysts [21, 25] or additives [29, 30] (e.g. PtCl2 or Al2O3) to promote the hydrochlorination. Others focus more on the kinetics and mechanism and have not achieved products in synthetically useful yields because of poor selectivity or low conversion [26-28]. Hence, hydrochlorination of terminal arylalkynes in the absence of any additives or catalysts has not been studied well. Herein, we report a mild, straightforward, highly regioselective, and catalyst-free method for the synthesis of vinyl chlorides through hydrochlorination of arylalkynes with HCl.
2. ExperimentalThe melting points were determined using a WRS-2 apparatus. The IR spectra were recorded on an Avatar 360 FT-IR spectrometer. The 1H (400 MHz) and 13C (100 MHz) NMR spectra of the samples were recorded on an AVANCE Ⅲ HD 400 spectrometer and calibrated to the residual solvent signals or the internal standard signal. MS (EI, 70 eV) experiments were performed on an Agilent 5973 N or GCT spectrometer. HRMS (EI) experiments were performed on a Waters Micromass GCT spectrometer. Synthesis of arylalkynes 1i-1k and the spectral data and spectra of all compounds are given in Supporting information.
General procedure for hydrochlorination: The arylalkyne 1, acetic anhydride (Ac2O), and an anhydrous solvent were added to a dried three-necked flask under argon atmosphere and the mixture was stirred for 30 min at room temperature. Further, HCl(g) prepared using H2SO4 and NaCl at 160 °C was added to the mixture through the rubber tube until the starting material conversed completely, monitored by TLC, and the unreacted HCl(g) was absorbed by aqueous NaOH. The solvent was removed and the residue was purified using flash chromatography on a silica gel.
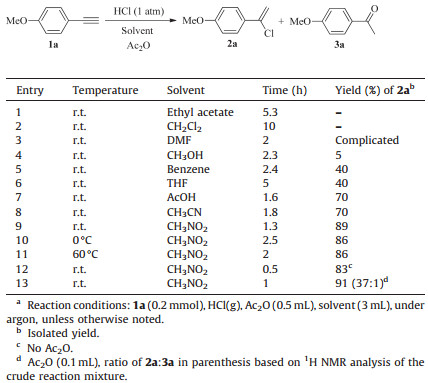
3. Results and discussionAccording to the literatures [26, 27], hydrochlorination was accompanied by hydration of alkynes with H2O. Thus, we used HCl(g) instead of aqueous HCl and Ac2O as the drier. To optimize the reaction conditions, 4-methoxy phenylacetylene 1a was chosen as the model substrate for hydrochlorination of arylalkynes with HCl in the presence of Ac2O. We observed that no reaction occurred when ethyl acetate and CH2Cl2 were used as the solvents, while a complicated mixture was produced when DMF was used (Table 1, entries 1-3). The 1-(1-chlorovinyl)-4-methoxybenzene 2a was isolated in a 5% yield when the reaction was conducted in CH3OH (Table 1, entry 4). To improve the reaction efficiency, we further screened benzene, THF, AcOH, CH3CN, and CH3NO2. CH3NO2 was found to be the best solvent, and using the other solvents resulted in lower yields of 2a in 40%-70% (Table 1, entries 5-9). To determine the optimized reaction temperature, the model reaction was carried out at different temperatures. It was observed that room temperature was best and lower and higher temperatures did not improve the yield of 2a (Table 1, entries 9-11). The control experiment showed that the addition of Ac2O is necessary, which can inhibit the formation of ketone through hydration of 1a; however, the amount of Ac2O has a marginal influence on the reaction because water was little bit in the reaction system (Table 1, entries 12-13).
|
|
Table 1 Optimization of the reaction conditions.a |
With the optimized reaction conditions in hand, we then investigated the scope of the hydrochlorination reaction of arylalkynes, and the results are summarized in Table 2. We initially explored the electronic effect of the substituents on the phenyl ring. Arylalkynes with strong electron-donating groups, such as 4-alkoxy, 4-dimethylamino, 4-acetamido, 4-benzamido, and 2-benzamido, were easily converted to their corresponding vinyl chlorides in good to excellent yields (75%-99%) (Table 2, entries 1, 6-12). While reactions of arylalkynes substituted by weak electron-donating groups such as 4-alkyl (Table 2, entries 13-15), proceeded slower and afforded the desired products in lower yields (60%-65%) than those with strong electron-donating groups. However, hydrochlorination of arylalkynes bearing strong electron-withdrawing groups, such as 4-cyano and 4-acetyl, did not proceed at all even after 38 h (Table 2, entries 18 and 19).These results indicate that the electronic effect of the substituents on the phenyl ring plays significant role in hydrochlorination of alkynes and the hydrochlorination mechanism has an electrophilic nature [31]. Furthermore, the relative position effect of the substituents on the phenyl ring was investigated. Arylalkynes possessing a methoxy at the 4-, 3-, or 2-position of the phenyl ring yielded the desired vinyl chlorides in 91%, 35%, and 85% yields (Table 2, entries 1-3), respectively, and reaction of 3-methyoxy phenylacetylene 1b proceeded slowly to generate the corresponding product in a poor yield (35%) owing to bad chemoselectivity. These results show that the position effect of the substituents also has a tremendous influence on hydrochlorination of alkynes. Subsequently, we investigated the steric effect. 2, 6-Dimethoxyphenylacetylene 1d produced the corresponding hydrochlorination product in an excellent yield (95%) (Table 2, entry 4), which reveals that a highly steric substrate is well tolerated in this reaction.The vinyl chlorides of Markovnikov addition were obtained exclusively in all cases.
|
|
Table 2 Hydrochlorination of arylalkynes with HCl.a |
4. Conclusion
We presented a simple and straightforward method for the synthesis of vinyl chlorides that exhibit unique regioselectivity through hydrochlorination of arylalkynes using HCl(g) under mild conditions. The advantage of this protocol is that the hydrochlorination works well in the absence of any additives or metal catalysts, and the protocol is simple and inexpensive and can potentially be applied to the methodologies for substrate preparation.
AcknowledgmentWe greatly acknowledge financial support from the National Basic Research Program of China (973 Program, No. 2012CB720300), the Applied Basic Research Program of Bingtuan (No. 2015AG001), and the High-level Talent Scientific Research Project of Shihezi University (No. RCZX201405).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.04.019.
| [1] | K. Kamei, N. Maeda, R. Ogino, New 5-HT1A receptor agonists possessing 1, 4-benzoxazepine scaffold exhibit highly potent anti-ischemic effects. Bioorg. Med. Chem. Lett. 11 (2001) 595–598. DOI:10.1016/S0960-894X(01)00008-7 |
| [2] | S. Nishikawa, M. Sato, H. Kojima, Convenient synthesis and cytokinin activity of β-substituted 4-styrylpyridines, the simplest cytokinin analogs with a moderate cell division-promoting activity. J. Agri. Food Chem. 44 (1996) 1337–1342. DOI:10.1021/jf9502313 |
| [3] | T. He, L.L. Wu, X.L. Fu, Copper and amine free Sonogashira cross-coupling reaction catalyzed by efficient diphosphane-palladium catalyst. Chin. Chem. Lett. 22 (2011) 1175–1178. |
| [4] | F. Xiao, Y. Xue, Y. Luo, Synthesis and cytotoxic activity of 7-alkynyl camptothecin derivatives. Chin. Chem. Lett. 20 (2009) 566–568. DOI:10.1016/j.cclet.2008.12.019 |
| [5] | S. Ma, X. Jiang, X. Cheng, H. Hou, Highly efficient Suzuki coupling reaction of achloroalkylidene-b-lactones and b-lactams with organoboronic acids. Adv. Synth. Catal. 348 (2006) 2114–2124. DOI:10.1002/(ISSN)1615-4169 |
| [6] | R. Rossi, F. Bellina, M. Lessi, Highly selective palladium-catalyzed Suzuki-Miyaura monocoupling reactions of ethene and arene derivatives bearing two or more electrophilic sites. Tetrahedron 67 (2011) 6969–7025. DOI:10.1016/j.tet.2011.06.001 |
| [7] | A. Thakur, K. Zhang, J. Louie, Suzuki-Miyaura coupling of heteroaryl boronic acids and vinyl chlorides. Chem. Commun. 48 (2012) 203–205. DOI:10.1039/C1CC15990A |
| [8] | K. Kamei, N. Maeda, T. Tatsuoka, A practical synthetic method for vinyl chlorides and vinyl bromides from ketones via the corresponding vinyl phosphate intermediates. Tetrahedron Lett. 46 (2005) 229–232. DOI:10.1016/j.tetlet.2004.11.075 |
| [9] | A. Spaggiari, D. Vaccari, P. Davoli, G. Torre, F. Prati, Amild synthesis of vinyl halides and gem-dihalides using triphenyl phosphite-halogen-based reagents. J. Org. Chem. 72 (2007) 2216–2219. |
| [10] | M. Kodomari, T. Nagaoka, Y. Furusawa, Convenient synthesis of aryl-substituted halo olefins from aromatic ketones and acetyl halides in the presence of silica gelsupported zinc halides. Tetrahedron Lett. 42 (2001) 3105–3107. DOI:10.1016/S0040-4039(01)00378-1 |
| [11] | W. Su, C. Jin, First catalytic and green synthesis of aryl-(Z)-vinyl chlorides and its plausible addition-elimination mechanism. Org. Lett. 9 (2007) 993–996. DOI:10.1021/ol062991c |
| [12] | K. Kokubo, K. Matsumasa, M. Miura, M. Nomura, Rhodium-catalyzed reaction of aroyl chlorides with alkynes. J. Org. Chem. 61 (1996) 6941–6946. DOI:10.1021/jo960915p |
| [13] | T. Kashiwabara, M. Tanaka, Rhodium-catalyzed addition of α-keto acid chlorides with terminal alkynes. Adv. Synth. Catal. 353 (2011) 1485–1490. DOI:10.1002/adsc.201100108 |
| [14] | T. Iwai, T. Fujihara, J. Terao, Y. Tsuji, Iridium-catalyzed addition of aroyl chlorides and aliphatic acid chlorides to terminal alkynes. J. Am. Chem. Soc. 134 (2012) 1268–1274. DOI:10.1021/ja209679c |
| [15] | G. Zhu, D. Chen, Y. Wang, R. Zheng, Highly stereoselective synthesis of (Z)-1, 2-dihaloalkenes by a Pd-catalyzed hydrohalogenation of alkynyl halides. Chem. Commun. 48 (2012) 5796–5798. DOI:10.1039/c2cc31553j |
| [16] | F. Yang, K.G. Ji, S. Ali, Y.M. Liang, Sc(OTf)3-catalyzed synthesis of indoles and SnCl4-mediated regioselective hydrochlorination of 5-(arylamino)pent-3-yn-2-ones. J. Org. Chem. 76 (2011) 8329–8335. DOI:10.1021/jo201514q |
| [17] | N.A. Petasis, I.A. Zavialov, Mild conversion of alkenyl boronic acids to alkenyl halides with halosuccinimides. Tetrahedron Lett. 37 (1996) 567–570. DOI:10.1016/0040-4039(95)02262-7 |
| [18] | Y. Masuda, M. Hoshi, A. Arase, Simple stereospecific syntheses of (E)-1-chloro(or bromo)alk-1-enes from alk-1-ynes via hydroboration. J. Chem. Soc. Perkin Trans. 1 (1992) 2725–2726. |
| [19] | G. Zweifel, W. Lewis, Stereoselective syntheses of ((E)-and (Z)-1-halo-1-alkenyl)silanes from alkynes. J. Org. Chem. 43 (1978) 2739–2744. DOI:10.1021/jo00408a001 |
| [20] | C. Xu, W. Du, Y. Zeng, B. Dai, H. Guo, Reactivity switch enabled by counterion: highly chemoselective dimerization and hydration of terminal alkynes. Org. Lett. 16 (2014) 948–951. DOI:10.1021/ol403684a |
| [21] | S. Dérien, H. Klein, C. Bruneau, Selective ruthenium-catalyzed hydrochlorination of alkynes: one-step synthesis of vinylchlorides. Angew. Chem. Int. Ed. 54 (2015) 12112–12115. DOI:10.1002/anie.201505144 |
| [22] | X. Li, Y. Wang, L. Kang, M. Zhu, B. Dai, A novel, non-metallic graphitic carbon nitride catalyst for acetylene hydrochlorination. J. Catal. 311 (2014) 288–294. DOI:10.1016/j.jcat.2013.12.006 |
| [23] | L. Wang, F. Wang, J. Wang, Enhanced stability of hydrochlorination of acetylene using polyaniline-modified Pd/HY catalysts. Catal. Commun. 74 (2016) 55–59. DOI:10.1016/j.catcom.2015.10.027 |
| [24] | K. Griesbaum, V.V.R. Rao, G. Leifker, Unusual products from the reactions of anhydrous hydrogen chloride with arylacetylenes. J. Org. Chem. 47 (1982) 4975–4981. DOI:10.1021/jo00146a029 |
| [25] | C.Y. Lo, M.P. Kumar, H.K. Chang, Regioselective haloaromatization of 1J. Chem. Soc. Perkin Trans.. J. Org. Chem. 70 (2005) 10482–10487. DOI:10.1021/jo0518295 |
| [26] | R.C. Fahey, D.J. Lee, Polar additions to olefins and actylenes. Ⅲ. The kinetics and stereochemistry of addition in the system 1-phenylpropyne-hydrogen chlorideacetic acid. J. Am. Chem. Soc. 88 (1966) 5555–5560. DOI:10.1021/ja00975a038 |
| [27] | R.C. Fahey, M.T. Payne, D.J. Lee, Reaction of acetylenes with hydrogen chloride in acetic acid. Effect of structure upon AdE2 and Ad3 reaction rates. J. Org. Chem. 39 (1974) 1124–1130. DOI:10.1021/jo00922a024 |
| [28] | R.C. Fahey, D.J. Lee, Polar additions to olefins and acetylenes. V. Bimolecular and termolecular mechanisms in the hydrochlorination of acetylenes. J. Am. Chem. Soc. 90 (1968) 2124–2131. DOI:10.1021/ja01010a034 |
| [29] | P.J. Kropp, K.A. Daus, S.D. Crawford, Surface-mediated reactions. 1. Hydrohalogenation of alkenes and alkynes. J. Am. Chem. Soc. 112 (1990) 7433–7434. DOI:10.1021/ja00176a075 |
| [30] | P.J. Kropp, S.D. Crawford, Surface-mediated reactions. 4. Hydrohalogenation of alkynes. J. Org. Chem. 59 (1994) 3102–3112. DOI:10.1021/jo00090a031 |
| [31] | F. Marcuzzi, G. Melloni, On the stereochemistry of the electrophilic addition of alkyl halides and hydrogen halides to phenyl-substituted acetylenes. J. Am. Chem. Soc. 98 (1976) 3295–3300. DOI:10.1021/ja00427a040 |
 2016, Vol. 27
2016, Vol. 27