Mechanically interlocked molecules (MIMs) have attracted tremendous attention due to their beautiful architectures, topological importance, and potential applications in constructing switches [1, 2], logic gates [3], sensors [4-7], drug delivery systems [8], artificial molecular machines [9-12], and surface materials [13, 14]. Among the various types of MIMs, pseudo[1]rotaxane [15] has been a research hotspot for its unique structure, where the wheel is threaded by its own axle, playing an important role in the MIM family. As the fundamental building blocks in fabricating complicated structures, so far many researchers have been conducted to explore their dynamic behaviors or potential applications [16, 17].
Due to the dynamic nature of pseudo[1]rotaxane, the external environment plays an essential role in the stability of this inclusion structure, for example, the polarity of the solvent [18], temperature [19], and other external stimuli [20-22], which greatly affect the binding affinity of the cyclic host molecules with the threaded guest molecules. As we know, solvent with strong polarity, such as DMSO, usually destroys the host-guest interaction, thus prohibit-ing the formation of pseudo[1]rotaxane structure. Up to now, various types of pseudo[1]rotaxanes have been reported in non-polar or weak polar solvents, especially in the pillar[n]arene family, where the polarity of the solvents greatly affected the pillararene-based host-guest interaction and played a vital role in the formation of pseudo[1]rotaxanes [23-29]. Although some reported pillar[n]arene-based pseudo[1]rotaxanes could partially exist in the mixed solution of non-polar solvent with partial DMSO [25, 29], the self-inclusion structures would be totally destroyed in DMSO solution. Therefore, the construction of stable pseudo[1]rotaxanes in strong polar solvent remains a challenging work, which usually needs reasonable structural design; and it is not only essential in investigating the formation process of pseudo[1]rotaxane, but also has potential applications in constructing artificial molecular machines.
Recently, we have synthesized a biotin modified pillar[5]arene-based pseudo[1]rotaxane [29], which could form a self-inclusion structure in chloroform or acetone, while such self-inclusion structure would be gradually destroyed by increasing the polarity of the solvent, exhibiting a slow-exchange process within NMR time scale. In order to further understand the self-assembly process of such pseudo[1]rotaxane, herein, a mono-functionalized pillar[5]arene derivative (P2) bearing alkyl chain as the ending group and short ethylene glycol (EGn) chain as flexible linker was synthesized. To our delight, it was found that a stable pseudo[1]r-otaxane could be formed in DMSO (Scheme 1), which also showed responsiveness to sodium cation. Subsequently, to further investigate the formation process of such pseudo[1]rotaxane, two similar pillar[5]arene derivatives (P1 and P3) bearing different length of EGn chains were synthesized. And the results showed that P1 bearing a shorter EGn chain than P2 could also form stable pseudo[1]rotaxane in DMSO, whereas, concentration-dependent pseudorotaxane structures were formed under the same condition by P3 which bearing a longer and more flexible EGn chain. This study indicated that the flexibility of the side chain plays a vital role in the formation of pseudo[1]rotaxanes.
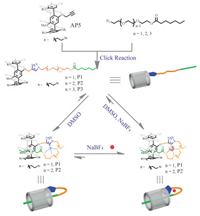
|
Download:
|
| Scheme. 1. Schematic illustration of the dynamic behavior of pseudo[1]roataxanes. | |
2. Experimental 2.1. General information
All reactions were performed in air atmosphere unless otherwise stated. The commercially available reagents and solvents were either employed as purchased or dried according to procedures described in the literature. Column chromatography was performed with silica gel (200-300 mesh) produced by Qingdao Marine Chemical Factory, Qingdao (China). All yields were given as isolated yields. NMR spectra were recorded on a Bruker DPX 300 MHz spectrometer (or Bruker DPX 400 MHz spectrome-ter) with internal standard tetramethylsilane (TMS) and solvent signals as internal references at 298 K, and the chemical shifts (δ) were expressed in ppm and J values were given in Hz. Low-resolution electrospray ionization mass spectra (LR-ESI-MS) were obtained on Finnigan Mat TSQ 7000 instruments. High-resolution electrospray ionization mass spectra (HR-ESI-MS) were recorded on an Agilent 6540Q-TOF LCMS equipped with an electrospray ionization (ESI) probe operating in positive-ion mode with direct infusion. Melting points (Mp) were determined using a Focus X-4 apparatus (made in China) and were not corrected.
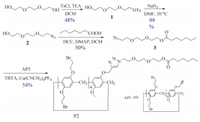
2.2. The synthetic procedures for mono-functionalized pillar[5]arene derivatives P2The detailed synthesis procedures for compounds 1-3 were provided in Supporting information.
P2: Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine (TBTA) (0.05 g, 0.1 mmol) and [Cu(CH3CN)4]PF6 (0.04 g, 0.1 mmol) were added to a solution of 3 (0.29 g, 1.0 mmol) and AP5 [29] (1.52 g, 1.0 mmol) in chloroform (20 mL) under argon atmosphere, and the mixture was stirred at 25 °C for 12 h (Scheme 2). The resulting solution was concentrated in vacuum, and the crude product was purified by column chromatography (dichloromethane) to afford the target molecule P2 as a white solid (1.10 g, 0.54 mmol, 54%). Mp 144 -146 °C. 1H NMR (300 MHz, DMSO-d6, 298 K): δ 8.16 (s, 1H, CH), 7.03 -6.79 (m, 10H, ArH), 5.00 (s, 2H, OCH2), 4.58 (t, 2H, J=4.3 Hz, NCH2), 4.29 -4.05 (m, 18H, OCH2), 3.73 -3.64 (m, 31H, ArCH2, BrCH2, OCH2, and OCH3), 3.49 -3.41 (m, 6H, OCH2), 1.84 (t, 2H, J=7.8 Hz, COCH2), 0.65 -0.53 (m, 7H, CH2 and CH3), -0.13 --0.20(m, 4H, CH2). 13C NMR (100 MHz, DMSO-d6, 298 K): δ 173.1, 150.1, 149.0, 148.94, 148.90, 148.8, 148.7, 143.0, 128.40, 128.35, 128.27, 128.1, 128.0, 127.8, 127.4, 124.2, 115.1, 114.8, 113.0, 69.5, 69.4, 68.6, 68.4, 68.3, 68.0, 62.9, 61.6, 55.47, 49.45, 33.3, 32.6, 32.3, 32.2, 32.1, 32.0, 29.9, 28.4, 27.3, 24.0, 21.8, 13.7. HR-ESI-MS: m/z Calcd. for C68H83Br8N3NaO + [M + Na]+ 1827.9152, found 1827.9158.
|
Download:
|
| Scheme. 2. The synthetic route of P2. | |
2.3. The synthesis of P1
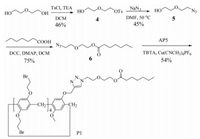
The detailed synthesis procedures for compounds 4-6 were provided in Supporting information.
P1: Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine (TBTA) (0.05 g, 0.1 mmol) and [Cu(CH3CN)4]PF6 (0.04 g, 0.1 mmol) were added to a solution of 6 (0.24 g, 1.0 mmol) and AP5 (1.52 g, 1.0 mmol) in chloroform (20 mL) under argon atmosphere, and the mixture was stirred at 25 °C for 12 h (Scheme 3). The resulting solution was concentrated in vacuum, and the crude product was purified by column chromatography (dichloromethane) to afford the target molecule P1 as a white solid (1.02 g, 0.54 mmol, 54%).Mp 165 -167 °C. 1H NMR (300 MHz, DMSO-d6, 298 K): δ 8.14 (s, 1H, NCH), 7.12 -6.82 (m, 10H, ArH), 5.01 (s, 2H, NCH2), 4.61 (t, 2H, J=4.7 Hz, OCH2), 4.20 -4.01 (m, 18H, OCH2), 3.86 -3.62 (m, 33H, ArCH2, OCH2, OCH3, and BrCH2), 1.85 -1.81 (m, 2H, COCH2), 0.62 (brs, 2H, CH2), 0.40 (brt, 3H, J=7.1 Hz, CH3), 0.27 (brd, 2H, J=7.3 Hz, CH2), -0.44 (brs, 4H, CH2). 13C NMR (100 MHz, DMSO-d6, 298 K): δ 173.1, 150.4, 149.02, 148.96, 148.9, 148.8, 148.7, 128.34, 128.25, 128.21, 128.12, 128.10, 127.99, 127.97, 127.5, 115.5, 115.0, 114.84, 114.81, 114.68, 114.66, 114.54, 114.53, 114.3, 112.9, 68.62, 68.57, 68.36, 68.29, 68.27, 68.23, 68.1, 68.0, 62.5, 55.3, 49.4, 33.4, 32.6, 32.2, 32.1, 32.0, 31.94, 31.92, 29.4, 28.8, 28.5, 28.4, 28.3, 27.2, 23.9, 21.7, 13.5. HR-ESI-MS: m/z Calcd. for C66H79Br8N3NaO13+ [M + Na]+ 1783.8890, found 1783.8904.
|
Download:
|
| Scheme. 3. The synthetic route of P1. | |
2.4. The synthesis of P3
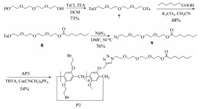
The detailed synthesis procedures for compounds 7-9 were provided in Supporting information.
P3: Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine (TBTA) (0.05 g, 0.1 mmol) and [Cu(CH3CN)4]PF6 (0.04 g, 0.1 mmol) were added to a solution of 9 (0.24 g, 0.72 mmol) and AP5 (1.52 g, 1.0 mmol) in chloroform (20 mL) under argon atmosphere, and the mixture was stirred at 25 °C for 12 h (Scheme 4). The resulting solution was concentrated in vacuo. The crude product was purified by column chromatography (dichloromethane) to afford the target molecule P3 as a white solid (1.10 g, 0.054 mmol, 54%). Mp 148 -149 °C. 1H NMR (400 MHz, DMSO-d6, 298 K): δ 8.18 (s, 1H, NCH), 7.06 -6.82 (m, 10H, ArH), 5.05 (s, 2H, NCH2), 4.57 (t, 2H, J=5.0 Hz, OCH2), 4.23 (brs, 13H, ArCH2, and OCH3), 4.14 -4.06 (m, 4H, OCH2), 3.86 -3.65 (m, 32H, BrCH2, and OCH2), 3.60 -3.34 (m, 10H, OCH2), 2.05 (t, 2H, J=7.7 Hz, COCH2), 1.09 -1.06 (m, 2H, CH2), 0.93 -0.88 (m, 2H, CH2), 0.69 (t, 3H, J=7.2 Hz, CH3), 0.56 (brs, 4H, CH2). 13C NMR (100 MHz, DMSO-d6, 298 K): δ 173.0, 150.0, 149.02, 149.01, 148.96, 148.92, 148.90, 148.84, 148.78, 148.5, 143.0, 128.5, 128.4, 128.2, 128.0, 127.9, 127.5, 124.4, 115.18, 115.16, 114.9, 114.8, 114.6, 114.5, 114.3, 113.7, 113.2, 69.7, 69.6, 69.54, 69.46, 68.7, 68.6, 68.44, 68.39, 68.3, 68.0, 62.9, 61.6, 55.5, 49.5, 33.4, 32.6, 32.3, 32.22, 32.16, 32.13, 30.3, 28.7, 28.45, 28.40, 27.6, 24.1, 21.9, 13.8. HR-ESI-MS: m/z Calcd. for C70H87Br8N3NaO15+ [M + Na]+ 1871.9415, found 1871.9421.
|
Download:
|
| Scheme. S4. The synthetic route of P3. | |
3. Results and discussion
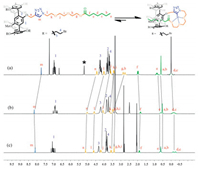
Initially, mono-functionalized pillar[5]arene derivative P2 was synthesized by means of copper (Ⅰ) catalyzed azide-alkyne cycloaddition [30]. The alkynyl-functionalized pillar[5]arene AP5 was synthesized according to reported literature [29]. Meanwhile, the azide-modified compound 3 was obtained by using commer-cially available triethylene glycol as the starting material (Scheme 2). It was obvious that in DMSO-d6 the protons on the alkyl chain (Ha-Hf) shifted upfield, indicating the inclusion of the alkyl chain into the cavity of the pillar[5]arene (Fig. 1b). Then, the self-complexation behavior of P2 in solution was studied by 1H NMR spectroscopy in different solvents. From the spectra of P2 (Fig. 1), obviously upfield chemical shifts of the protons (Ha-f) on the alkyl chain could be observed in nonpolar solvent (chloroform), weak polar solvent (acetone), and polar solvent (DMSO), which suggested that the solvent polarity had little effect on the inclusion behavior of P2. Moreover, from the 2D NOESY spectroscopy in DMSO-d6, strong NOE correlations between protons (Ha-f) on the alkyl chain and the protons (H1) on phenyl rings of pillar[5]arene could be observed (Fig. 2), further indicating that the alkyl chain was threaded into the cavity of pillar[5]arene. The above results were very different from those previous reports on pseudo[1]r-otaxanes that were formed from mono-functionalized pillar[n]ar-enes, where solvent with strong polarity would completely destroy the inclusion structures [23, 29]. However, in our system, stable inclusion structures could be formed in high polar solvent (DMSO) and exhibited a dynamic fast-exchange process within the NMR time scale.
|
Download:
|
| Figure 1. 1H NMR spectra (400 MHz, 298 K) of P2 (5 mmol/L) in different solvents: (a) CDCl3, (b) DMSO-d6, and (c) acetone-d6. ("*" indicates the solvent residue). | |
|
Download:
|
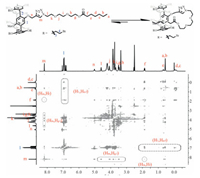
| Figure 2. Partial 2D NOESY spectrum of P2 (400 MHz, DMSO-d6, 298 K). | |
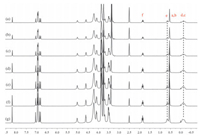
To investigate whether this inclusion behavior was intermo-lecular or intramolecular, variable concentration 1H NMR spec-troscopy of P2 at different concentrations in the range of 7.0-80 mmol/L was carried out in DMSO-d6 (Fig. 3), which showed that the inclusion-structure formed by P2 was concentration indepen-dent, suggesting that P2 exists predominantly in the form of pseudo[1]rotaxane. The above result was also supported by the theoretical equation of self-inclusion equilibrium in solution (Supporting information). Subsequently, high resolution electro-spray ionization mass spectrometry (HR-ESI-MS) investigation was performed, in which one indicative peak corresponding to [M + Na]+ at m/z 1827.9158 was clearly observed and no other peaks corresponding to higher molecular weight interlocked structures could be observed, confirming the presence of P2 as the pseudo[1]rotaxane structure in DMSO [31]. Moreover, strong NOE correlations between the proton on triazole group (Hm) and the protons (Hg-l) on EGn units could also be observed (Fig. 2), indicating that intramolecular hydrogen-bonding interaction between the proton on the triazole ring and the oxygen atoms on the EGn chain was exist, which might be the main driving force for the formation of the self-inclusion structure.
|
Download:
|
| Figure 3. 1H NMR spectra (300 MHz, 298 K) of P2 at variant concentrations in DMSO-d6 indicate its concentration-independent property: (a) 7 mmol/L, (b) 15 mmol/L, (c) 20 mmol/L, (d) 25 mmol/L, (e) 40 mmol/L, (f) 60 mmol/L, and (g) 80 mmol/L. | |
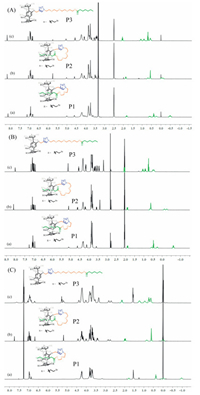
This interesting and abnormal phenomenon had greatly attracted our attention. To further investigate the formation process of this type of pseudo[1]rotaxane, another two pillar[5]-arene derivatives bearing shorter (P1) or longer (P3) EGn chain as flexible linker were also synthesized by click reaction. From their 1H NMR spectra in different solvents (Fig. 4), obvious upfield chemical-shifts of the protons on the alkyl chain of P1 could be observed in chloroform, acetone as well as DMSO, indicating the formation of inclusion structure in these solvents. Moreover, this inclusion structure formed from P1 could also be confirmed by 2D NOESY analysis based on the NOE correlation signals between the protons on the alkyl side chain and pillar[5]arene cavity (Fig. S25 in Supporting information). However, with respect to P3 which bearing longer and more flexible side chain, only slight upfield chemical shifts could be observed from the 1H NMR spectra of P3 in nonpolar solvent (chloroform), weak polar solvent (acetone) as well as polar solvent (DMSO), indicating the tendency of forming unthreaded structures in dilute solution. Moreover, comparing the 1H NMR spectra of P1, P2, and P3 in DMSO-d6 (Fig. 4A), it was observed that the protons on the alkyl chain of P1 exhibited the largest upfield shifts, while those alkyl chain protons of P3 showed the smallest upfield shifts. And similar phenomena could also be observed in both chloroform and acetone (Fig. 4B and Fig. 4C). The above difference might be caused by the flexibility of the side chain, which would hinder the inclusion tendency of the alkyl side chain due to its random movement.
|
Download:
|
| Figure 4. 1H NMR spectra of P1, P2, and P3: (A) 7 mmol/L, 400 MHz, DMSO-d6, 298 K; (B) 7 mmol/L, 400 MHz, acetone-d6, 298 K; (C) 3 mmol/L, 400 MHz, CDCl3, 298 K. | |
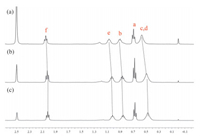
In the following study, variable concentration experiments in DMSO suggested that the inclusion-structure formed from P1 was also concentration independent (Fig. 5), indicating the formation of pseudo[1]rotaxane similar to that of P2. Whereas, P3 exhibited different result: as shown in Fig. 6, the 1H NMR spectra of P3 in DMSO-d6 at different concentrations of 10, 20, and 50 mmol/L were recorded, and it was found that the protons on the alkyl chain gradually shifted upfield upon increasing the concentration, which meant the inclusion structure formed by P3 was concentration-depended, suggesting the formation of other concentration-dependent pseudorotaxane structures in high concentration.

|
Download:
|
| Figure 5. 1H NMR spectra (400 MHz, 298 K) of P1 at variant concentrations in DMSO-d6: (a) 10 mmol/L, (b) 50 mmol/L. | |
|
Download:
|
| Figure 6. Partial 1H NMR spectra (400 MHz, 298 K) of P3 at variant concentrations in DMSO-d6 indicate its concentration-dependent property: (a) 10 mmol/L, (b) 20 mmol/L, and (c) 50 mmol/L. | |
Subsequently, study of host-guest chemistry between pillar[5]-arene and alkyl chain was carried out in DMSO-d6 by using 1, 4-dimethoxypillar[5]arene (DMP5) as the model host molecule and azide-modified compound 6 as guest molecule (Fig. 7). From the 1H NMR spectra of DMP5 with 10 equiv. of guest compound 6, it was found that the chemical shifts of protons on DMP5 almost did not show any changes, indicating that the host-guest interaction was too weak in DMSO. The above results also demonstrated that pillararene-based host-guest interaction might not be the main driving force for such inclusion behavior.

|
Download:
|
| Figure 7. 1H NMR spectra (300 MHz, DMSO-d6, 298 K) of (a) DMP5 (5 mmol/L), (b) 6 (50 mmol/L) + DMP5 (5 mmol/L), (c) 6 (50 mmol/L). ("*" indicates the solvent residue). | |
On the basis of the above observations and our previous work on the biotin functionalized pseudo[1]rotaxane [29], we could draw the conclusion that the intramolecular hydrogen-bonding interaction might play a vital role in the formation of pseudo[1]r-otaxane, which can make the side chain bend back to thread into its own cavity; whereas, pillararene-based host-guest interaction had almost no contribution to the pseudo[1]rotaxane formation in polar solvent; moreover, the flexibility of the side chain might affect its inclusion tendency thus hinder the formation of pseudo[1]rotax-anes, so mono-functionalized pillar[5]arenes with longer and flexible side chain will lead to the generation of concentration-dependent pseudorotaxane.
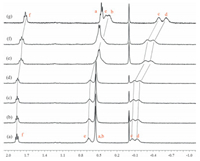
After understanding the formation process of such pseudo[1]r-otaxanes, we further tended to investigate their dynamic behaviors in polar solution. As we know, most pillararene-based host-guest interactions will be destroyed in high polar solution, such as DMSO, so adding competitive guests might have no effect on this self-inclusion structure. Inspired by the host-guest interaction between crown ethers with alkaline metal cation [32], we wondered whether such pseudo[1]rotaxane structures would show responsiveness to sodium cation due to the presence of electronegative EGn chains. Along this line, NaBF4 was added gradually to the P2 solution in DMSO-d6. From the 1H NMR spectra (Fig. 8), it was observed that the protons on the alkyl chain exhibited slight upfield shifts upon gradually adding Na+, indicating the alkyl chain showed a stronger tendency to thread into the cavity of pillar[5]arene. Meanwhile, protons Hm and Hnthat belong to the triazole and its neighboring groups showed the same upfield changing tendency (Fig. S27 in Supporting informa-tion), which might result from the destroy of the hydrogen bonds between the triazole ring and ethylene glycol chain, thus led to the generation of Na+-induced more tightening self-inclusion struc-ture. Moreover, similar results could also be observed by adding NaBF4 to the solution of P1 (Fig. 9).
|
Download:
|
| Figure 8. Partial 1H NMR spectra (400 MHz, 298 K, DMSO-d6) of P2 (5 mmol/L) with different equivalents of NaBF4: (a) 0 equiv., (b) 1.0 equiv., (c) 2.0 equiv., (d) 5.0 equiv., (e) 30.0 equiv., (f) 35.0 equiv., and (g) 60.0 equiv. | |

|
Download:
|
| Figure 9. 1H NMR spectra (400 MHz, 298 K, DMSO-d6) of P1 (5 mmol/L) with different equivalents of NaBF4: (a) 0 equiv., (b) 40 equiv. | |
4. Conclusion
In summary, mono-alkyl-functionalized pillar[5]arenes (P1, P2, and P3) bearing different length of EGn chains as flexible linkers were synthesized in a facial way. Among which P1 and P2 exhibited self-inclusion behavior in strong polar solvent DMSO, leading to the generation of stable pseudo[1]rotaxanes; whereas, P3 with longer and more flexible side chain led to the formation of concentration-dependent pseudorotaxane structures at high con-centration in DMSO. Furthermore, the pseudo[1]rotaxane formed by P1 or P2 exhibited stimuli-responsiveness to Na+, resulting in the formation of more tightening inclusion structures, which might be driven by the interaction between the positively charged Na+ and electronegative EGn chain. The discovery of stable pseudo[1]rotaxanes formed in polar solvent represents a special type of inclusion phenomenon, and further studies on the controllable switch of pseudo[1]rotaxanes and their applications are now ongoing in our lab.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21472089, 21572101), and the National Natural Science Foundation of Jiangsu (No. BK20140595).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.05.004.
| [1] | C.J. Bruns, J.F. Stoddart, Rotaxane-based molecular muscles. Acc. Chem. Res. 47 (2014) 2186–2199. DOI:10.1021/ar500138u |
| [2] | L. Liu, Y. Liu, P. Liu, Phosphine oxide functional group based three-station molecular shuttle. Chem. Sci. 4 (2013) 1701–1706. DOI:10.1039/c3sc22048f |
| [3] | A. Credi, V. Balzani, S.J. Langford, J.F. Stoddart, Logic operations at the molecular level. An XOR gate based on a molecular machine. J. Am. Chem. Soc. 119 (1997) 2679–2681. DOI:10.1021/ja963572l |
| [4] | C. Gao, X. Ma, Q. Zhang, A light-powered stretch-contraction supramolecular system based on cobalt coordinated[1] rotaxane. Org. Biomol. Chem. 9 (2011) 1126–1132. DOI:10.1039/C0OB00764A |
| [5] | L. Liu, Q. Wang, M. Cheng, A ferrocene-functionalized bistable [2] rotaxane with switchable fluorescence. Asian J. Org. Chem. 4 (2015) 221–225. DOI:10.1002/ajoc.201402155 |
| [6] | M. Gangopadhyay, A.K. Mandal, A. Maity, Tuning emission responses of a triphenylamine derivative in host-guest complexes and an unusual dynamic inclusion phenomenon. J. Org. Chem. 81 (2016) 512–521. DOI:10.1021/acs.joc.5b02353 |
| [7] | A.K. Mandal, M. Suresh, M.K. Kesharwani, Molecular interactions, proton exchange, and photoinduced processes prompted by an inclusion process and a [2] pseudorotaxane formation. J. Org. Chem. 78 (2013) 9004–9012. DOI:10.1021/jo400752d |
| [8] | R. Barat, T. Legigan, I. Tranoy-Opalinski, A mechanically interlocked molecular system programmed for the delivery of an anticancer drug. Chem. Sci. 6 (2015) 2608–2613. DOI:10.1039/C5SC00648A |
| [9] | J.D. Badjic, V. Balzani, A. Credi, S. Silvi, J.F. Stoddart, A molecular elevator. Science 302 (2004) 1845–1849. |
| [10] | B. Lewandowski, G. De Bo, J.W. Ward, Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339 (2013) 189–193. DOI:10.1126/science.1229753 |
| [11] | S. Erbas-Cakmak, D.A. Leigh, C.T. McTernan, A.L. Nussbaumer, Artificial molecular machines. Chem. Rev. 115 (2015) 10081–10206. DOI:10.1021/acs.chemrev.5b00146 |
| [12] | S.H. Li, H.Y. Zhang, X. Xu, Y. Liu, Mechanically selflocked chiral gemini-catenanes. Nat. Commun. 6 (2015) 7590–7596. DOI:10.1038/ncomms8590 |
| [13] | S. Saha, K.C.F. Leung, T.D. Nguyen, J.F. Stoddart, J. Zink, Nanovalves. Adv. Funct. Mater 17 (2007) 685–693. DOI:10.1002/(ISSN)1616-3028 |
| [14] | A.C. Fahrenbach, S.C. Warren, J.T. Incorvati, Organic switches for surfaces and devices. Adv. Mater. 25 (2013) 331–348. DOI:10.1002/adma.201201912 |
| [15] | D.B. Amabilino, J.F. Stoddart, Interlocked and intertwined structures and superstructures. Chem. Rev. 95 (1995) 2725–2828. DOI:10.1021/cr00040a005 |
| [16] | D.H. Qu, Q.C. Wang, Q.W. Zhang, X. Ma, H. Tian, Photoresponsive host-guest functional systems. Chem. Rev. 115 (2015) 7543–7588. DOI:10.1021/cr5006342 |
| [17] | M. Xue, Y. Yang, X. Chi, X. Yan, F. Huang, Development of pseudorotaxanes and rotaxanes: from synthesis to stimuli-responsive motions to applications. Chem. Rev. 115 (2015) 7398–7501. DOI:10.1021/cr5005869 |
| [18] | D.B. Smithrud, E.M. Sanford, I. Chao, Solvent effects in molecular recognition. Pure Appl. Chem. 62 (1990) 2227–2236. |
| [19] | P.R. Ashton, I. Baxter, M.C.T. Fyfe, Rotaxane or pseudorotaxane? That is the question!. J. Am. Chem. Soc. 120 (1998) 2297–2307. DOI:10.1021/ja9731276 |
| [20] | M. Ni, Y. Guan, L. Wu, Improved recognition of alkylammonium salts by ion pair recognition based on a novel heteroditopic pillar [5] arene receptor. Tetrahedron Lett. 53 (2012) 6409–6413. DOI:10.1016/j.tetlet.2012.09.043 |
| [21] | G. Yu, C. Han, Z. Zhang, Pillar [6] arene-based photoresponsive host-guest complexation. J. Am. Chem. Soc. 134 (2012) 8711–8717. DOI:10.1021/ja302998q |
| [22] | Y. Inoue, P. Kuad, Y. Okumura, Thermal and photochemical switching of conformation of poly(ethylene glycol)-substituted cyclodextrin with an azobenzene group at the chain end. J. Am. Chem. Soc. 129 (2007) 6396–6397. DOI:10.1021/ja071717q |
| [23] | Y. Chen, D. Cao, L. Wang, Monoester copillar [5] arenes: synthesis, unusual self-inclusion behavior, and molecular recognition. Chem. Eur. J. 19 (2013) 7064–7070. DOI:10.1002/chem.v19.22 |
| [24] | Y. Guan, P. Liu, C. Deng, Dynamic self-inclusion behavior of pillar [5] arenebased pseudo[1] rotaxanes. Org. Biomol. Chem. 12 (2014) 1079–1089. DOI:10.1039/c3ob42044b |
| [25] | M. Ni, X.Y. Hu, J. Jiang, L. Wang, The self-complexation of mono-ureafunctionalized pillar [5] arenes with abnormal urea behaviors. Chem. Commun. 50 (2014) 1317–1319. DOI:10.1039/C3CC47823H |
| [26] | T. Ogoshi, K. Demachi, K. Kitajima, T.A. Yamagishi, Monofunctionalized pillar [5] -arenes: synthesis and supramolecular structure. Chem. Commun. 47 (2011) 7164–7166. DOI:10.1039/c1cc12333e |
| [27] | N.L. Strutt, H. Zhang, M.A. Giesener, J. Lei, J.F. Stoddart, A self-complexing and selfassembling pillar [5] arene. Chem. Commun. 48 (2012) 1647–1649. DOI:10.1039/C2CC16030G |
| [28] | C.L. Sun, J.F. Xu, Y.Z. Chen, Monofunctionalized pillar [5] arene-based stable[1] pseudorotaxane. Chin. Chem. Lett. 26 (2015) 843–846. DOI:10.1016/j.cclet.2015.05.030 |
| [29] | X. Wu, M. Ni, W. Xia, X.Y. Hu, L. Wang, A novel dynamic pseudo[1] rotaxane based on a mono-biotin-functionalized pillar [5] arene. Org. Chem. Front. 2 (2015) 1013–1017. DOI:10.1039/C5QO00159E |
| [30] | K.D. Hanni, D.A. Leigh, The application of CuAAC 'click' chemistry to catenane and rotaxane synthesis. Chem. Soc. Rev. 39 (2010) 1240–1251. DOI:10.1039/B901974J |
| [31] | K. Wang, C.Y. Wang, Y. Zhang, Ditopic pillar [5] arene-based fluorescence enhancement material mediated by[c2] daisy chain formation. Chem. Commun. 50 (2014) 9458–9461. |
| [32] | S.K. Kim, J.L. Sessler, Calix [4] pyrrole-based ion pair receptors. Acc. Chem. Res. 47 (2014) 2525–2536. DOI:10.1021/ar500157a |
 2016, Vol. 27
2016, Vol. 27 


