b The Second Affiliated Hospital of Lanzhou University, Lanzhou 730000, China
In recent years, biodegradable and biocompatible poly (lactic acid) (PLA) has received widespread attention and has been widely applied in medical and pharmaceutical industries [1]. Ring-opening polymerization (ROP) is a convenient method to synthesize PLA with well-controlled molecular weight and low polydispersity (PDI) under mild condition [2]. Many metal such as aluminum [3], magnesium [4], zinc [5], indium [6], lanthanide [7], and other transition metals [8] complexes have been synthesized and successfully used as catalyst for ROP of lactide. Many of these complexes have been excellent initiators/catalysts for the ROP of lactide. As we known, metallic residues in polymers were difficult to remove. Therefore, nontoxic metal complexes including zinc, magnesium, sodium and potassium [9] have been widely used in ROP. Among them, many zinc complexes were used for the ROP of lactide due to their high Lewis acidity and biocompatible.
Due to the Schiff base ligands were diversity and easy to synthesis, a lot of zinc complexes supported by Schiff base ligands were synthesized and applied to catalyze the ROP of lactide with excellent catalytic performance [10]. For example, zinc silylamido complexes can be effective initiators for the controlled ROP of rac-LA and exhibited high stereoselectivity (Ps=0.80-0.84) [11]. However, reports of zinc complexes supported by two bis-ligated Schiff base ligands were not explored widely [12].
Melt phase ROP was an effective method to afford polymers with high molecular weight, which was particularly relevant to industry [13]. Various metal complexes have been used for lactide ROP in the melt state [14]. However, there are very few reports to explore the catalytic performance of catalysts/initiators based on nontoxic metals [15]. Thus, in this context a series of zinc complexes supported by two bis-ligated Schiff base ligands and application in rac-LA polymerization in solution and in molten lactide was reported.
2. Experimental 2.1. General preparation procedures for HL1-HL6Amines (2 mmol), salicyaldehydes (2 mmol), and a catalytic amount of AcOH in methanol were combined and refluxed overnight. Upon cooling the resulting reaction solution to ambient temperature, yellow crystals precipitated from solution, the solid product was filtered and washed with cold methanol and then dried in vacuo to give the desired ligands.
Synthesis of HL1: The HL1 was obtained by reaction of 9H-xanthene-9-amine and 2-hydroxy-3, 5-di-tert-butylbenzaldehyde. Yield 0.31 g (37.5%) 1H NMR (300 MHz, CDCl3, ppm): 13.29 (s, 1H, Ar-OH), 8.44 (s, 1H, Ar-CHN), 7.39-7.08 (m, 10H, Ar-H), 5.76 (s, 1H, (Ar)2-CHN), 1.38 (s, 9H, C(CH3)3), 1.31 (s, 9H, C(CH3)3); 13C NMR (75 MHz, CDCl3, ppm) 165.04, 157.98, 150.98, 140.24, 136.79, 129.33, 129.18, 127.40, 126.46, 123.32, 120.76, 117.55, 116.86, 63.22, 35.00, 34.15, 31.48, 29.37.
Synthesis of HL2: The HL2 was obtained by reaction of 9-aminoanthracene and 2-hydroxy-3, 5-di-tert-butylbenzaldehyde. Yield 0.33 g (40.3%) 1H NMR (300 MHz, CDCl3, ppm): 13.56 (s, 1H, Ar-OH), 8.44 (s, 1H, Ar-CHN), 8.31 (s, 1H, Ar-H), 8.09 (d, 1H, J=9.0 Hz, Ar-H), 8.02 (d, 2H, J=6.0 Hz, Ar-H), 7.58 (d, 1H, J=3.0 Hz, Ar-H) 7.52-7.42 (m, 4H, Ar-H), 7.24 (d, 1H, J=3.0 Hz, Ar-H), 1.57 (s, 9H, C(CH3)3), 1.36 (s, 9H, C(CH3)3); 13C NMR (75 MHz, CDCl3, ppm) 170.25, 158.58, 142.95, 140.80, 137.36, 131.74, 128.63, 128.25, 127.08, 125.52, 125.48, 123.76, 123.42, 122.90, 117.97, 35.26, 34.24, 31.47, 29.53.
Synthesis of HL3: The HL3 was obtained by reaction of 9H-xanthene-9-amine and 2-hydroxy-5-tert-butylbenzaldehyde. Yield 0.38 g (45.3%) 1H NMR (300 MHz, CDCl3, ppm): 13.97 (s, 1H, Ar-OH), 8.01 (s, 1H, Ar-CHN), 7.41 (dd, 1H, J=6.0 Hz, Ar-H), 7.37-7.20 (m, 15H, Ar-H), 7.10 (d, 1H, J=3.0 Hz, Ar-H), 6.98 (d, 1H, J=9.0 Hz, Ar-H), 1.28 (s, 9H, C(CH3)3); 13C NMR (75 MHz, CDCl3, ppm) 165.16, 158.90, 144.55, 141.38, 130.12, 129.66, 128.62, 128.03, 127.20, 118.03, 116.60, 33.99, 31.41.
Synthesis of HL4: The HL4 was obtained by reaction of triphenylmethylamine and 2-hydroxy-5-tert-butylbenzaldehyde. Yield 0.36 g (50.4%) 1H NMR (300 MHz, CDCl3, ppm): 12.77 (s, 1H, Ar-OH), 8.40 (s, 1H, Ar-CHN), 7.37-7.08 (m, 10H, Ar-H), 6.87 (d, 1H, J=9.0 Hz, Ar-H), 5.78 (s, 1H, (Ar)2-CHN), 1.30 (s, 1H, C(CH3)3); 13C NMR (75 MHz, CDCl3, ppm) 164.34, 258.65, 151.01, 141.45, 130.06, 129.23, 129.18, 128.26, 123.36, 120.66, 117.74, 116.88, 116.63, 63.08, 33.99, 31.42.
Synthesis of HL5: The HL5 was obtained by reaction of triphenylmethylamine and 5-chlorosalicylaldehyde. Yield 0.41 g (51.6%) 1H NMR (300 MHz, CDCl3, ppm): 14.12 (s, 1H, Ar-OH), 7.91 (s, 1H, Ar-CHN), 7.37-7.15 (m, 16H, Ar-H), 7.11 (d, 1H, J=3.0 Hz, Ar-H), 6.96 (d, 1H, J=6.0 Hz, Ar-H); 13C NMR (75 MHz, CDCl3, ppm) 163.71, 159.73, 144.01, 132.48, 131.38, 129.55, 128.12, 127.40, 123.25, 119.48, 118.58, 79.21.
Synthesis of HL6: The HL6 was obtained by reaction of 9H-xanthene-9-amine and 5-chlorosalicylaldehyde. Yield 0.32 g (47.8%) 1H NMR (300 MHz, CDCl3, ppm): 12.98 (s, 1H, Ar-OH), 8.25 (s, 1H, Ar-CHN), 7.37-7.10 (m, 10H, Ar-H), 6.84 (d, 1H, J=9.0 Hz, Ar-H), 5.83 (s, 1H, (Ar)2-CHN); 13C NMR (75 MHz, CDCl3, ppm) 162.47, 159.43, 151.10, 132.39, 130.94, 129.47, 129.19, 123.48, 123.26, 120.02, 119.26, 118.57, 117.00, 62.91.
2.2. General preparation procedures for complexes 1-6Under an inert atmosphere, a ZnEt2 solution (0.12 mmol, 1 mol/L in n-hexane) was added dropwise into n-hexane solution (10 mL) of appropriate ligand HL1-HL6 (0.2 mmol). The reaction mixture was stirred overnight. The solid product was filtered and recrystallized from n-hexane solution affording a crystalline powder.
Synthesis of 1: Yield. 0.042 g (47.3%). 1H NMR (300 MHz, CDCl3, ppm): 7.82 (s, 1H, Ar-CHN), 7.37 (d, 1H, J=3.0 Hz, Ar-H), 7.25-7.18 (m, 3H, Ar-H), 7.11-7.06 (m, 3H, Ar-H), 6.96-6.88 (m, 2H, Ar-H), 6.62 (d, 1H, J=3.0 Hz, Ar-H), 5.67 (s, 1H, (Ar)2-CHN), 1.30 (s, 1H, C(CH3)3), 1.26 (s, 1H, C(CH3)3); 13C NMR (100 MHz, CDCl3, ppm) 172.01, 169.10, 151.72, 141.47, 134.90, 130.00, 129.70, 129.19, 123.90, 123.64, 120.36, 119.88, 116.85, 61.48, 35.43, 33.77, 31.34, 29.51; Elemental analysis (%) calcd. for C56H60N2O4Zn: C 75.53, H 6.79, N 3.15; found: C 75.27, H 6.82, N 3.05.
Synthesis of 2: Yield. 0.035 g (39.8%). 1H NMR (300 MHz, CDCl3, ppm): 8.40 (d, 1H, J=9.0 Hz, Ar-H), 8.03 (s, 1H, Ar-CHN), 7.95 (s, 1H, Ar-H), 7.94 (d, 1H, J=9.0 Hz, Ar-H), 7.55-7.38 (m, 4H, Ar-H), 6.98-6.95 (m, 1H, Ar-H), 6.70 (d, 1H, J=3.0 Hz, Ar-H), 6.22 (d, 2H, J=3.0 Hz, Ar-H), 1.63 (s, 1H, C(CH3)3), 1.20 (s, 1H, C(CH3)3); 13C NMR (100 MHz, CDCl3, ppm) 176.61, 170.52, 142.40, 141.94, 135.31, 131.30, 131.19, 130.84, 129.94, 127.90, 127.63, 126.26, 125.62, 125.17, 124.93, 124.71, 124.53, 124.40, 123.89, 120.94, 116.55, 35.74, 33.79, 31.11, 29.60; Elemental analysis (%) calcd. for C58H60N2O2Zn: C 78.94, H 6.85, N 3.17; found: C 78.64, H 6.76, N 3.35.
Synthesis of 3: Yield. 0.032 g (36%). 1H NMR (300 MHz, CDCl3, ppm): 7.85 (s, 1H, Ar-CHN), 7.22-7.08 (m, 16H, Ar-H), 6.48-6.43 (m, 2H, Ar-H), 1.21 (s, 1H, C(CH3)3); 13C NMR (100 MHz, CDCl3, ppm) 177.51, 168.89, 145.61, 135.85, 133.28, 130.73, 130.03, 128.03, 126.63, 123.28, 116.91, 80.88, 33.51, 31.35; Elemental analysis (%) calcd. for C60H56N2O2Zn: C 79.85, H 6.25, N 3.10; found: C 79.55, H 6.22, N 3.29.
Synthesis of 4: Yield. 0.033 g (43%). 1H NMR (300 MHz, CDCl3, ppm): 7.80 (s, 1H, Ar-CHN), 7.33 (d, 1H, J=6.0 Hz, Ar-H), 7.20-6.95 (m, 8H, Ar-H), 6.75 (d, 1H, J=3.0 Hz, Ar-H), 6.55 (d, 1H, J=6.0 Hz, Ar-H), 5.50 (s, 1H, (Ar)2-CHN), 1.31 (s, 1H, C(CH3)3); 13C NMR (100 MHz, CDCl3, ppm) 169.30, 168.77, 151.43, 136.29, 133.02, 131.08, 129.72, 129.49, 123.38, 123.20, 119.41, 116.87, 116.42, 62.97, 33.59, 31.40; Elemental analysis (%) calcd. for C48H44N2O4Zn: C 74.08, H 5.70, N 3.60; found: C 74.28, H 5.92, N 3.55.
Synthesis of 5: Yield. 0.041 g (48%). 1H NMR (300 MHz, CDCl3, ppm): 7.79 (s, 1H, Ar-CHN), 7.22-7.14 (m, 15H, Ar-H), 7.03 (dd, 1H, J=9.0 Hz, Ar-H), 6.62 (d, 1H, J=3.0 Hz, Ar-H), 6.42 (d, 1H, J=6.0 Hz, Ar-H); 13C NMR (100 MHz, CDCl3, ppm) 176.50, 169.01, 145.14, 135.04, 133.78, 129.87, 128.24, 127.12, 125.17, 118.18, 117.54, 81.24; Elemental analysis (%) calcd. for C52H38Cl2N2O2Zn: C 72.69, H 4.46, N 3.26; found: C 72.54, H 4.41, N 3.28.
Synthesis of 6: Yield. 0.029 g (40%). 1H NMR (300 MHz, CDCl3, ppm): 7.77 (s, 1H, Ar-CHN), 7.28-6.88 (m, 12H, Ar-H), 6.44 (d, 1H, J=6.0 Hz, Ar-H), 5.33 (s, 1H, (Ar)2-CHN); 13C NMR (100 MHz, CDCl3, ppm) 169.19, 167.33, 151.67, 134.84, 133.91, 130.01, 129.60, 125.25, 123.67, 118.87, 117.90, 117.74, 117.21, 63.62; Elemental analysis (%) calcd. for C40H26Cl2N2O4Zn: C 65.37, H 3.57, N 3.81; found: C 65.19, H 3.27, N 3.70.
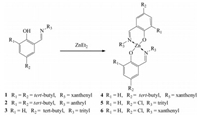
3. Results and discussionIn order to study the influence of steric hindrance and electronic effect of catalyst, ligands HL1-HL6 were synthesized by the reaction of different amine with related 2-hydroxybenzaldehyde derivatives under refluxing methanol. Reaction of these ligands with ZnEt2 in a 2:1 molar ratio afforded the zinc complexes 1-6 in 37%-52% yield (Scheme 1). All of the obtained complexes were confirmed by 1H, 13C NMR and elemental analysis.
|
Download:
|
| Scheme. 1. Synthesis of complexes 1-6. | |
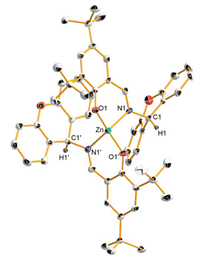
Complex 1 was further characterized by single crystal X-ray diffraction [16]. Single crystal of complex 1 suitable for the structural characterization was grown at ambient temperature from a saturated THF/n-hexane solution. As the ORTEP drawing shown in Fig. 1, two bidentate N, O-ligands coordinate to the central metal ion in distorted tetrahedron geometry, but they seemed to be not symmetrical due to two different orientations of two xanthenyl groups. However, the NMR spectra showed one set of signals for complex 1 in either 1H NMR or 13C NMR (Figs. S3 and S4 in Supporting information), for example, there are same proton signals at 5.67 ppm for H1 and H1'. This can be interpreted as two xanthenyl groups of complex 1 can rotate freely around the single bonds of C1-N1 and C1' -N1'.
|
Download:
|
| Figure 1. ORTEP drawing of complex 1 with probability ellipsoids at the 30%, most of hydrogen atoms are omitted for clarity. | |
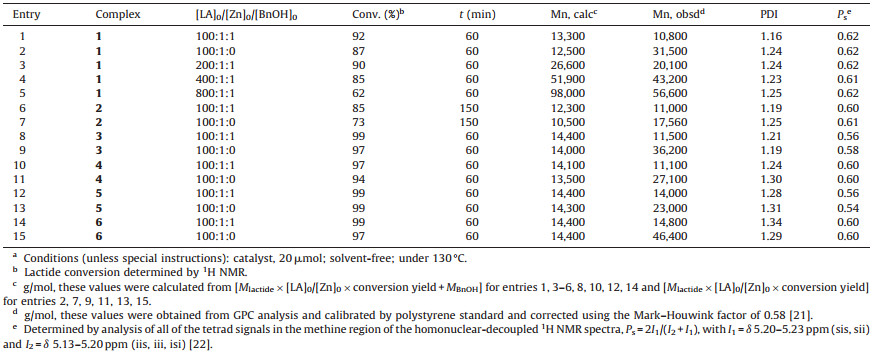
The results for rac-lactide ROP in the melt catalyzed by complexes 1-6 were listed in Table 1. Treatment of rac-LA with complexes in a 100:1 molar ratio without benzyl alcohol (BnOH) as co-initiator, the polymerization of rac-LA within 60 min for complexes 1 and 3-6 and 150 min for complex 2 can be almost finished, but the molecular weights are unpredictable. Upon one equivalent of BnOH was added to the system ([monomer]:[cata-lyst]=100:1), the molecular weights (Mn) of obtained polymers become close to the theoretical molecular weight and the rate of polymerization also increases. This means that the nucleophilicity of the phenolate ligand is weaker than the benzyl alkoxide group which is generated via the activation BnOH by the phenoxy group of these complexes [17]. Comparatively, the activity of complex 2 is the lowest because the special hindrance of anthryl group is very big; complex 4 showed higher activity than complex 1 (conver-sion: 97% vs. 92%) under the same polymerization conditions (entries 1 and 10, Table 1), this can be ascribed to that the bulky tert-butyl group of the complex 1 can inhibit the coordination of lactide to the metal center. The rate of polymerization for complex 6 and 4 were slightly slower than 5 and 3 respectively (entries 8-15, Table 1) possibly because the steric hindrance of xanthenyl group was bigger than trityl group. Hetero-selectivity of complexes 3 and 5 were lower than that of other complexes, indicating that the trityl groups on the ligands not favorable to hetero-selectivity. Obviously, complex 1 showed slightly better hetero-selectivity and controllability than other complexes, so complex 1 was used as the best catalyst for the next research. The side reaction became serious when the ratio of [monomer]:[catalyst] increase up to 800 because the molecular weight of resulting PLA deviate far from the theoretical molecular weight (entry 5, Table 1).
|
|
Table 1 Ring-opening polymerization of rac-lactide catalyzed by complexes 1-6.a |
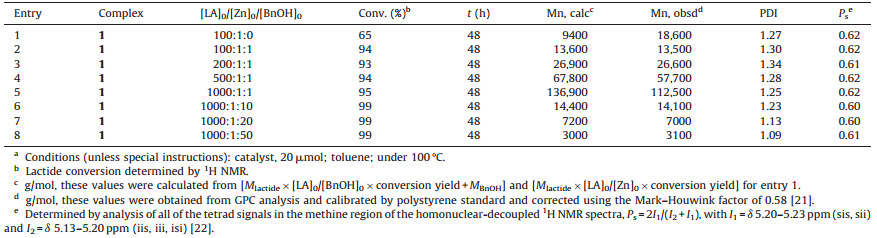
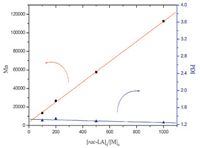
Complex 1 also was tested for the ROP of rac-LA in toluene under 100 °C. As shown in Table 2, when the ratio of 100:1 of [rac-LA]0:[Zn]0 was used for the polymerization without or with one equivalent of BnOH as co-initiator, the polymerization of rac-LA within 48 h can reached 65% and 94% conversion (entries 1 and 2, Table 2), respectively. Although the reaction requires 48 h to reach completion and the hetero-selectivity of complex 1 remained low, the molecular weights of resulting PLAs almost consist with the theoretical molecular weights. In addition, the linear relationships between the molecular weights of the polymers and the monomer-to-initiator ratio indicate a living polymerization catalyzed by complex 1 (Fig. 2). In particular, the polymer obtained by polymerization of 1000 equivalents of rac-LA for complex 1 also possesses a desirable molecular weight (entry 5, Table 2). The polymer generated by complex 1 with a desirable molecular weight and narrow PDI in the presence of 10, 20, and 50 equivalents of BnOH (entries 6-8, Table 2) indicate that complex 1 can catalyze the polymerization of rac-lactide under a controllable manner with an immortal character.
|
|
Table 2 Polymerization of rac-lactide by complex 1 in toluene.a |
|
Download:
|
Figure 2. The linear relationships between the Mn(  |
|
Compared to other similar zinc complexes reported for ROP of rac-LA [18], complexes 1-6 showed better control performance with living and immortal character. It is worth mentioning that the molecular weight of resulting PLA was close to the theoretical molecular weight even for the polymerization of 1000 equivalents of rac-LA catalyzed by complex 1. However, the catalytic activity and selectivity of complexes were less than some recently reported zinc catalysts [18, 19].
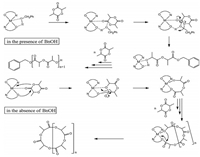
1H NMR spectrum of the polymer obtained via the reaction of complex 1, rac-LA, and BnOH in the melt was shown in Fig. 3, the invariant complex 1 and PLA were obtained by the reaction of rac-LA with complex 1 and BnOH in a 10:1:1 ratio under 130 °C, the 1H NMR spectrum shown peaks at 7.33 ppm (He), 4.36 ppm (Hc), and 2.70 ppm (Hf) with a ratio close to 5:1:1, which indicate that the obtained polymer was capped with one benzyl ester and one hydroxyl group. Thus, the activated monomer mechanism was feasible mechanism for this polymerization process (Fig. 4) [20].

|
Download:
|
| Figure 3. 1H NMR spectrum of the product obtained from the reaction of complex 1, rac-LA and BnOH (1 stand for complex 1, [LA]0/[Zn]0/[BnOH]0=10:1:1, 130 °C, 1 h). | |
|
Download:
|
| Figure 4. Proposed mechanism for catalytic ROP of rac-LA using complexes 1-6. | |
No terminal groups were observed in the 1HNMR spectroscopy of the PLA obtained under melting conditions without BnOH as initiator provide cyclic polymers were obtained (Fig. S25 in Supporting information). It is further confirmed by ESI-MS of the PLA (Fig. S26 in Supporting information), two series of main peaks at 72 m + 39 and 72 m + 23 can be ascribed to m (C3H4O2) + K+ and m (C3H4O2) + Na+, respectively. The series of peaks with a difference of 72 m/z, indicated that some transesterification reactions also occurred during the polymerization process. As already mentioned above, the polymerization process should be initiated by the phenolate ligand nucleophilic attack to the monomer as shown in Fig. 4 [17].
4. ConclusionIn summary, bis-ligated zinc complexes 1-6 can efficiently catalyze the polymerization of rac-LA to obtain PLAs in solution and melt conditions. The controllability of this system became better in the presence of BnOH. Complex 1 even can catalyze the ROP of 1000 equivalents of rac-lactide with desirable molecular weight. The steric hindrance and electronic effect have a great influence on the catalytic performance of these complexes.
AcknowledgmentThis work was supported by National Natural Science Founda-tion of China (Nos. 21271092, 21171078, and 21401161) and the Science Foundation of Gansu Province of China (No. 1308RJ2A121).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.05.001.
| [1] |
(a) D. Sykes, M.D. Ward, Visible-light sensitisation of Tb(Ⅲ) luminescence using a blue-emitting Ir(Ⅲ) complex as energy-donor, Chem. Commun. 47(2011) 2279-2281; (b) M. Labet, W. Thielemans, Synthesis of polycaprolactone: a review, Chem. Soc. Rev. 38(2009) 3484-3504; (c) A. Kowalski, A. Duda, S. Penczek, Mechanism of cyclic ester polymerization initiated with Tin(Ⅱ) octoate. 2. Macromolecules fitted with Tin(Ⅱ) alkoxide species observed directly in MALDI-TOF spectra, Macromolecules 33(2000) 689-695. |
| [2] |
(a) P.J. Dijkstra, H.Z. Du, J. Feijen, Single site catalysts for stereoselective ringopening polymerization of lactides, Polym. Chem. 2(2011) 520-527; (b) J.-C. Buffet, J. Okuda, Initiators for the stereoselective ring-opening polymerization of meso-lactide, Polym. Chem. 2(2011) 2758-2763; (c) M.J. Stanford, A.P. Dove, Stereocontrolled ring-opening polymerisation of lactide, Chem. Soc. Rev. 39(2010) 486-494. |
| [3] |
(a) X. Pang, R.L. Duan, X. Li, et al., Synthesis and characterization of half-salen complexes and their application in the polymerization of lactide and e-caprolactone, Polym. Chem. 5(2014) 6857-6864; (b) Z.H. Tang, X.S. Chen, X. Pang, et al., Stereoselective polymerization of raclactide using a monoethylaluminum Schiff base complex, Biomacromolecules 5(2014) 965-970; (c) A. Pilone, K. Press, I. Goldberg, et al., Gradient isotactic multiblock polylactides from aluminum complexes of chiral salalen ligands, J. Am. Chem. Soc. 136(2014) 2940-2943; (d) M. Normand, T. Roisnel, J.-F. Carpentier, E. Kirillov, Dinuclear vs. mononuclear complexes: accelerated, metal-dependent ring-opening polymerization of lactide, Chem. Commun. 49(2013) 11692-11694; (e) W. Zhao, Y. Wang, X.L. Liu, et al., Protic compound mediated living crosschain-transfer polymerization of rac-lactide: synthesis of isotactic (crystalline)-heterotactic (amorphous) stereomultiblock polylactide, Chem. Commun. 48(2012) 6375-6377. |
| [4] |
(a) T. Han, R. Petrus, D. Bykowski, L. Jerzykiewicz, P. Sobota, Synthesis and structural characterization of magnesium drug complexes: efficient initiators for forming polylactide-drug conjugates, Organometallics 34(2015) 4871-4880; (b) H.Y. Xie, Z.H. Mou, B. Liu, et al., Phosphinimino-amino magnesium complexes: synthesis and catalysis of heteroselective ROP of rac-lactide, Organometallics 33(2014) 722-730; (c) Y. Gao, Z. Dai, J. Zhang, X. Ma, N. Tang, J. Wu, Trinuclear and tetranuclear magnesium alkoxide clusters as highly active initiators for ring-opening polymerization of L-lactide, Inorg. Chem. 53(2013) 716-726; (d) W. Yi, H.Y. Ma, Magnesium and calcium complexes containing biphenylbased tridentate iminophenolate ligands for ring-opening polymerization of raclactide, Inorg. Chem. 52(2013) 11821-11835; (e) B.M. Chamberlain, M. Cheng, D.R. Moore, et al., Polymerization of lactide with zinc and magnesium b-diiminate complexes: stereocontrol and mechanism, J. Am. Chem. Soc. 123(2001) 3229-3238. |
| [5] |
(a) Y. Yang, H.B. Wang, H.Y. Ma, Stereoselective polymerization of rac-lactide catalyzed by zinc complexes with tetradentate aminophenolate ligands in different coordination patterns: kinetics and mechanism, Inorg. Chem. 54(2015) 5839-5854; (b) H.B. Wang, Y. Yang, H.Y. Ma, Stereoselectivity switch between zinc and magnesium initiators in the polymerization of rac-lactide: different coordination chemistry, different stereocontrol mechanisms, Macromolecules 47(2014) 7750-7764; (c) Z.R. Dai, J.J. Zhang, Y. Gao, et al., Synthesis and structures of tridentate bdiketiminato zinc phenoxides as catalysts for immortal ring-opening polymerization of L-lactide, Catal. Sci. Technol. 3(2013) 3268-3277; (d) P.D. Knight, A.J.P. White, C.K. Williams, Dinuclear zinc complexes using pentadentate phenolate ligands, Inorg. Chem. 47(2008) 11711-11719. |
| [6] |
(a) D.C. Aluthge, E.X. Yan, J.M. Ahn, P. Mehrkhodavandi, Role of aggregation in the synthesis and polymerization activity of salBinap indium alkoxide complexes, Inorg. Chem. 53(2014) 6828-6836; (b) I. Yu, A. Acosta-Ramírez, P. Mehrkhodavandi, Mechanism of living lactide polymerization by dinuclear indium catalysts and its impact on isoselectivity, J. Am. Chem. Soc. 134(2012) 12758-12773; (c) A. Pietrangelo, M.A. Hillmyer, W.B. Tolman, Stereoselective and controlled polymerization of D, L-lactide using indium(Ⅲ) trichloride, Chem. Commun. 19(2009) 2736-2737; (d) A.F. Douglas, B.O. Patrick, P. Mehrkhodavandi, A highly active chiral indium catalyst for living lactide polymerization, Angew. Chem. Int. Ed. 47(2008) 2290-2293. |
| [7] |
(a) C. Bakewell, A.J.P. White, N.J. Long, C.K. Williams, Metal-size influence in isoselective lactide polymerization, Angew. Chem. Int. Ed. 53(2014) 9226-9230; (b) C.G. Jaffredo, Y. Chapurina, S.M. Guillaume, J.-F. Carpentier, From syndiotactic homopolymers to chemically tunable alternating copolymers: highly active yttrium complexes for stereoselective ring-opening polymerization of b-malolactonates, Angew. Chem. Int. Ed. 53(2014) 2687-2691; (c) L.Clark, M.G. Cushion, H.E.Dyer, et al., Dicationicandzwitterionic catalysts for the amine-initiated, immortal ring-opening polymerisation of rac-lactide: facile synthesis of amine-terminated, highly heterotactic PLA, Chem. Commun. 46(2010) 273-275; (d) J.W. Kramer, D.S. Treitler, E.W. Dunn, et al., Polymerization of enantiopure monomers using syndiospecific catalysts: a new approach to sequence control in polymer synthesis, J. Am. Chem. Soc. 131(2009) 16042-16044; (e) H.Y. Ma, T.P. Spaniol, J. Okuda, Highly heteroselective ring-opening polymerization of rac-lactide initiated by bis(phenolato)scandium complexes, Angew. Chem. Int. Ed. 45(2006) 7818-7821. |
| [8] |
(a) C. Bakewell, G. Fateh-Iravani, D.W. Beh, et al., Comparing a series of 8-quinolinolato complexes of aluminium, titanium and zinc as initiators for the ringopening polymerization of rac-lactide, Dalton Trans. 44(2015) 12326-12337; (b) A. Stopper, K. Press, J. Okuda, I. Goldberg, M. Kol, Zirconium complexes of phenylene-bridged {ONSO} ligands: coordination chemistry and stereoselective polymerization of rac-lactide, Inorg. Chem. 53(2014) 9140-9150. |
| [9] |
(a) H.-W. Ou, K.-H. Lo, W.-T. Du, et al., Synthesis of sodium complexes supported with NNO-tridentate Schiff base ligands and their applications in the ringopening polymerization of L-lactide, Inorg. Chem. 55(2016) 1423-1432; (b) Z.R. Dai, Y.Y. Sun, J. Xiong, et al., Simple sodium and potassium phenolates as catalysts for highly isoselective polymerization ofrac-lactide, Catal. Sci. Technol. 6(2016) 515-520; (c) F.M. García-Valle, R. Estivill, C. Gallegos, et al., Metal and ligand-substituent effects in the immortal polymerization of rac-lactide with Li, Na, and K phenoxoimine complexes, Organometallics 34(2015) 477-487; (d) H.-Y. Chen, J.B. Zhang, C.-C. Lin, J.H. Reibenspiesa, S.A. Miller, Efficient and controlled polymerization of lactide under mild conditions with a sodium-based catalyst, Green Chem. 9(2007) 1038-1040. |
| [10] |
(a) D.J. Darensbourg, O. Karroonnirun, Ring-opening polymerization of lactides catalyzed by natural amino-acid based zinc catalysts, Inorg. Chem. 49(2010) 2360-2371; (b) D.J. Darensbourg, O. Karroonnirun, Ring-opening polymerization of L-lactide and e-caprolactone utilizing biocompatible zinc catalysts. Random copolymerization of L-lactide and e-caprolactone, Macromolecules 43(2010) 8880-8886; (c) H.-Y. Chen, H.-Y. Tang, C.-C. Lin, Ring-opening polymerization of lactides initiated by zinc alkoxides derived from NNO-tridentate ligands, Macromolecules 39(2006) 3745-3752. |
| [11] | M. Huang, C. Pan, H.Y. Ma, Ring-opening polymerization of rac-lactide and amethyltrimethylene carbonate catalyzed by magnesium and zinc complexes derived from binaphthyl-based iminophenolate ligands. Dalton Trans. 44 (2015) 12420–12431. DOI:10.1039/C5DT00158G |
| [12] | D.J. Darensbourg, P. Rainey, J. Yarbrough, Bis-salicylaldiminato complexes of zinc examination of the catalyzed epoxide/CO2 copolymerization. Inorg. Chem. 40 (2001) 986–993. DOI:10.1021/ic0006403 |
| [13] | A.C. Silvino, D.B. de Abreu Talina Martins, A. da Costa Rodrigues, M.L. Dias, Kinetic behavior in melt state and solid state polymerization of lactide using magnesium stearate as catalyst. J. Polym. Environ. 21 (2013) 1002–1008. DOI:10.1007/s10924-013-0603-1 |
| [14] |
(a) G.Q. Xiao, B. Yan, R. Ma, et al., Bulk ring-opening polymerization (ROP) of Llactide catalyzed by Ni(Ⅱ) and Ni(Ⅱ)-Sm(Ⅲ) complexes based on a salen-type Schiff-base ligand, Polym. Chem. 2(2011) 659-664; (b) A.D. Schwarz, Z.Y. Chu, P. Mountford, Sulfonamide-supported aluminum catalysts for the ring-opening polymerization of rac-lactide, Organometallics 29(2010) 1246-1260; (c) M. Bouyahyi, E. Grunova, N. Marquet, et al., Aluminum complexes of fluorinated dialkoxy-diimino salen-like ligands: syntheses, structures, and use in ringopening polymerization of cyclic esters, Organometallics 27(2008) 5815-5825; (d) A.J. Chmura, M.G. Davidson, C.J. Frankis, M.D. Jones, M.D. Lunn, Highly active and stereoselective zirconium and hafnium alkoxide initiators for solvent-free ring-opening polymerization of rac-lactide, Chem. Commun. 11(2008) 1293-1295. |
| [15] | K. Devaine-Pressing, J.H. Lehr, M.E. Pratt, Magnesium amino-bis(phenolato) complexes for the ring-opening polymerization of rac-lactide. Dalton Trans. 44 (2015) 12365–12375. DOI:10.1039/C5DT00236B |
| [16] | Crystallographic data for 1: C56H60N2O4Zn, M=888.38, crystal dimensions 0.170.180.23 mm3, triclinic, space group, P-1, a=10.9901(7), b=16.2430(19), c=17.7061(19)Å, V=2669.6(6)Å3, Z=2, calcd=1.2137 g cm-3, MoKa radiation (=0.71073Å), T=291 K. Scans, wR2=0.2141, R=0.0750, S=1.077, for 619 parameters. CCDC 1469815 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. |
| [17] |
(a) S.-C. Roşca, D.-A. Roşca, V. Dorcet, et al., Alkali aminoether-phenolate complexes: synthesis, structural characterization and evidence for an activated monomer ROP mechanism, Dalton Trans. 42(2013) 9361-9375; (b) R.K. Dean, A.M. Reckling, H. Chen, et al., Ring-opening polymerization of cyclic esters with lithium amine-bis(phenolate) complexes, Dalton Trans. 42(2013) 3504-3530; (c) N. Ikpo, C. Hoffmann, L.N. Dawe, F.M. Kerton, Ring-opening polymerization of e-caprolactone by lithium piperazinyl-aminephenolate complexes: synthesis, characterization and kinetic studies, Dalton Trans. 41(2012) 6651-6660. |
| [18] |
(a) Z.H. Mou, B. Liu, M.Y. Wang, et al., Isoselective ring-opening polymerization of rac-lactide initiated by achiral heteroscorpionate zwitterionic zinc complexes, Chem. Commun. 50(2014) 11411-11414; (b) K.A. Gerling, N.M. Rezayee, A.L. Rheingold, D.B. Greena, J.M. Fritsch, Synthesis and structures of bis-ligated zinc complexes supported by tridentate ketoimines that initiate L-lactide polymerization, Dalton Trans. 43(2014) 16498-16508; (c) S. Abbina, G.D. Du, Zinc-catalyzed highly isoselective ring opening polymerization of rac-lactide, ACS Macro Lett. 3(2014) 689-692. |
| [19] |
(a) M. Honrado, A. Otero, J. Fernández-Baeza, et al., Stereoselective ROP of raclactide mediated by enantiopure NNO-Scorpionate zinc initiators, Organometallics 33(2014) 1859-1866; (b) Y. Wang, H.Y. Ma, Exploitation of dinuclear salan aluminum complexes for versatile copolymerization of e-caprolactone and L-lactide, Chem. Commun. 48(2012) 6729-6731. |
| [20] |
(a) Z.R. Dai, Y.Y. Sun, J. Xiong, X.B. Pan, J.C. Wu, Alkali-metal monophenolates with a sandwich-type catalytic center as catalysts for highly isoselective polymerization of rac-lactide, ACS Macro Lett. 4(2015) 556-560; (b) H.-Y. Chen, L. Mialon, K.A. Abboud, S.A. Miller, Comparative study of lactide polymerization with lithium, sodium, magnesium, and calcium complexes of BHT, Organometallics 31(2012) 5252-5261; (c) A.K. Sutar, T. Maharana, S. Dutta, C.-T. Chen, C.-C. Lin, Ring-opening polymerization by lithium catalysts: an overview, Chem. Soc. Rev. 39(2010) 1724-1746. |
| [21] | A. Kowalski, A. Duda, S. Penczek, Polymerization of L, L-lactide initiated by aluminum isopropoxide trimer or tetramer. Macromolecules 31 (1998) 2114–2122. DOI:10.1021/ma971737k |
| [22] | L.F. Sánchez-Barba, A. Garcés, J. Fernández-Baeza, Stereoselective production of poly(rac-lactide) by ROP with highly efficient bulky heteroscorpionate alkylmagnesium initiators. Organometallics 30 (2011) 2775–2789. DOI:10.1021/om200163t |
 2016, Vol. 27
2016, Vol. 27