PP/PS blends, a type of commonly-known immiscible polymer blends, have been prepared by a variety of methods, including melt blending, reactor alloy processes, nucleation modification, in-situ polymerization, etc. [1-8]. PP/PS blends prepared byconventional melt mixing are macrophase-separated and not useful in practice, therefore reducing the size of PS particles and enhancing its compatibility with PP is a key issue for preparing useful PP/PS blend materials. Diffusion and subsequent polymerization of a monomer in commercial polymer pellets is a viable approach to prepare polymer blend pellets having very small dispersed phase size [9-13]. The average particle size is around or slightly higher than 100 nm. Various monomers or monomer mixtures such as styrene (St), butyl methacrylate (BMA) and mixtures of St/BMA have been successfully diffused into iPP pellets, and PP/PS, PP/ PBMA and PP/P(St-BMA) blend pellets were formed upon polymerization of the monomers. An important advantage of these blend pellets is their applicability for direct use in plastic production. As a versatile approach for quasi-nanoblends, improving its technology process can make it more suitable for industry, although a few methods for preparing nanoblends have been reported [8, 14-21]. A problem of concern is the polymer inevitably synthesized on the pellet surface for two reasons: (1) it can cause caking of the pellets d uring polymerization of the monomer and (2) micro-sized dispersed phase will be formed during processing. Therefore, control of surface morphology of the blend pellets by avoiding polymerization of the monomer on the pellet surface is highly necessary.
It is known that a small amount of inhibitor hydroquinone (HQ) is commonly added to commercial monomers for the purpose of stable storage of the monomers. This indicates that HQ can effectively catch up radicals and prevent polymerization of hydrophobic monomers such as styrene although it is only slightly soluble in hydrophobic monomers. In this work, a tiny amount of HQ is added to the reaction mixture just after the addition of initiator BPO in diffusion and subsequent polymerization of styrene in isotactic polypropylene pellets to prevent polymerization of styrene on the surface of iPP pellets. The procedures with or without HQ are compared in Fig. 1.
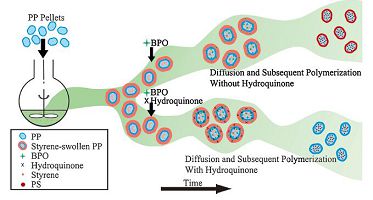
|
Download:
|
| Figure 1. Illustration of the diffusion and subsequent polymerization with (bottom) and without (top) hydroquinone | |
2. Experimental
IPP pellets (S1003) were from Yanshan Petroleum and Chemical, China. A typical procedure is as follows: iPP pellets (20.0 g), deionized water (52.5 mL) and aqueous polyvinyl alcohol (7.5 mL, 0.8 wt%) as a suspending agent were mixed by a mechanical stirrer in a 250 mL flask equipped with a condenser. The mixture was heated and maintained at 90 ℃. Styrene (6 g, 30 wt% to iPP) was added to the flask. After stirring for 2 h, the initiator BPO (0.06 g, 1 wt% to styrene) dissolved in 0.5 mL of chloroform and the inhibitor HQ dissolved in 1 mL of hot water were added and the polymerization proceeded at 90 ℃ for 3 h under stirring. After cooling, the blend pellets were filtrated, washed and dried to a constant weight.
Films for microscopic Fourier transform infrared spectroscopy spectra (Micro-FTIR) and with a thickness of 15 μm were obtained by cutting through the center of iPP/PS blend pellets by a microtome and swept with Thermo-Nicolet 6700 FT-IR equipped with Nicolet Continuum FT-IR Microscope along diameter direction with a step length of 100 μm.
Cross sections of single pellet for FESEM were obtained using the same cutting procedure as for Micro-FTIR, then etched for 1 h with stirring in a mixture of water (5 g), sulfuric acid (6 g, 95%), orthophosphoric acid (9 g, 85%) and potassium permanganate (0.08 g) to remove the amorphous regions of iPP and washed ultrasonically in water for 5 min and then in acetone for another 5 min before drying [9]. The morphology of the samples was visualized with a JSM-7401 field emission scanning electron microscope (FESEM). Particle sizes were counted with the aid of the Smile View software.
3. Results and discussionThe amount of HQis of key importance in view of the depth of HQ penetration and consequently the non-polymerized depth. Fig. 2 shows the distributions of PS along the diameter direction of the pellets obtained by Micro-FTIR. When a tiny amount of HQ (1/16×nBPO, i.e., 1/16 molar ratio to BPO) was used, very little PS appears in the subcutaneous layer of about 150 μm, and PS is almost evenly distributed in the other area of the pellets, indicating that HQ can indeed prevent polymerization of styrene on the surface of iPP pellets. However, more HQ can surprisingly increase the non- polymerized depth. When the molar amount of HQ equals that of BPO, the non-polymerization depth is 1000 μm and one large peak was observed in the central area of the pellet, indicating that HQcan diffuse into the pellets up to a depth of 1000 μm through the diffusion paths of St and St only polymerizes in the central area of the pellet where there is almost no HQ. With intermediate amounts of HQ, the non-polymerization depth is in the intermediate values, revealing that the penetration depth increases with increased amount of HQ. The formation of the two peaks can be explained by polymerization-induced diffusion of St from other places (non- polymerized region and the center) into the peak area once polymerization has started, and the St newly-diffused into the area polymerizes later on, form ing two maxima of PS/PP.

|
Download:
|
| Figure 2. Diametrical distribution of PS in PP/PS blend pellets synthesized with different amount of HQ(A1/A2 is defined as the ratio of the peak areas at 758 cm-1 and 1167 cm-1, characteristics of the PS and iPP, respectively) | |
The surface morphology of the blend pellets obtained with a tiny amount of HQ (1/16×nBPO) was investigated by FESEM and compared with those of virgin PP pellets and blend pellets obtained without using any HQ. As shown in Fig. 3, there are indeed PS blocks of various sizes on the surface of the blend pellets obtained without HQ (Fig. 3b). After washing with chloroform (Fig. 3c), the surface morphology is back to the original state (Fig. 3a), confirming the existence of PS blocks on the blend pellet surface. As predicted, there are almost no PS particles on the surface of the blend pellets obtained with HQ (Fig. 3d), confirming the effectiveness of HQin preventing polymerization of St on the pellet surface.

|
Download:
|
| Figure 3. FESEM micrographs: (a) surface of the original iPP pellet; surface of the PP/PS pellet prepared by diffusion and subsequent polymerization without HQ(b) and that after removal of the surface PS(c); (d) surface of the PP/PS pellet prepared by diffusion and subsequent polymerization with HQ | |
The phase morphology inside the blend pellets obtained with HQ was examined by FESEM. Fig. 4 shows phase morphology at 600 μm distance to the edge of the pellet and at the center of the pellet. Similar to the phase morphology of the blend pellets without HQ reported previously [9], fine morphology was observed for both sites with a lot of nano-sized PS particles. The average particle sizes are 146 nm and 85 nm for the two sites.

|
Download:
|
| Figure 4. FESEM micrographs of the cross section of PP/PS blend pellet synthesized with 1/16×nBPO: (a) 600 μm to the edge and (b) in the central area | |
4. Conclusion
The addition of a tiny amount of inhibitor hydroquinone can effectively inhibit polymerization of St on the surface of iPP pellets and at the same time maintain the fine phase morphology and homogeneous distribution of PS inside the pellets during the preparation of iPP/PS blend pellets by the method of diffusion and subsequent polymerization of styrene in iPP pellets, making an improvement in the preparation process for quasi-nanoblends.
AcknowledgmentThe authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 51173095).
| [1] | J.K. Lee, C.D. Han. Evolution of polymer blend morphology during compounding in a twin-screw extruder. Polymer 41 (2000) 1799–1815. DOI:10.1016/S0032-3861(99)00325-0 |
| [2] | J. Gao, X.T. Fu, M.M. Ding, Q. Fu. Studies on partial compatibility of PP and PS. Chin. J. Polym. Sci. 28 (2010) 647–656. DOI:10.1007/s10118-010-9150-6 |
| [3] | M. Rätzsch, M. Arnold, E. Borsig, H. Bucka, N. Reichelt. Radical reactions on polypropylene in the solid state. Prog. Polym. Sci. 27 (2002) 1195–1282. DOI:10.1016/S0079-6700(02)00006-0 |
| [4] | Z.Q. Su, M. Dong, Z.X. Guo, J. Yu. Study of polystyrene and acrylonitrile-styrene copolymer as special b-nucleating agents to induce the crystallization of isotactic polypropylene. Macromolecules 40 (2007) 4217–4224. DOI:10.1021/ma0623587 |
| [5] | Z.Q. Su, X.N. Chen, Z.Z. Yu, L. Zhang. Morphological distribution of polymeric nucleating agents in injection-molded isotactic polypropylene plates and its influence on nucleating efficiency. J. Appl. Polym. Sci. 111 (2009) 786–793. |
| [6] | L. Cao, M. Dong, A.Y. Zhang, et al. Morphologies and crystal structures of styreneacrylonitrile/isotactic polypropylene ultrafine fibers fabricated by melt electrospinning. Polym. Eng. Sci. 53 (2013) 2674–2682. DOI:10.1002/pen.v53.12 |
| [7] | Q. Li, X.Y. Zhang, J.F. Li, et al. A highly effective reactive liquid crystal for the improved b-nucleation of isotactic polypropylene. Polym. Eng. Sci. 54 (2014) 2112–2120. DOI:10.1002/pen.v54.9 |
| [8] | Z.M. Liu, Z.X. Dong, B.X. Han, et al. Composites prepared by the polymerization of styrene within supercritical CO2-swollen polypropylene. Chem. Mater. 14 (2002) 4619–4623. DOI:10.1021/cm0203215 |
| [9] | X.R. Yao, J. Yu, Z.X. Guo. Preparation of isotactic polypropylene/polystyrene blends by diffusion and subsequent polymerization of styrene in isotactic polypropylene pellets. Polymer 52 (2011) 667–675. DOI:10.1016/j.polymer.2010.12.050 |
| [10] | X.R. Yao, F. Chen, Z.X. Guo, J. Yu. Polypropylene/poly(butyl methacrylate) blends prepared by diffusion and subsequent polymerization of butyl methacrylate in isotactic polypropylene pellets. Chin. Chem. Lett. 23 (2012) 753–756. DOI:10.1016/j.cclet.2012.03.031 |
| [11] | X.R. Yao, Z.X. Guo, J. Yu. Preparation of PP/POE/PS ternary alloys via diffusion and polymerization of styrene in PP/POE alloys. Chem. J. Chin. Univ. 33 (2012) 2573–2578. |
| [12] | X.R. Yao, L. Wang, Z.X. Guo, J. Yu. Morphology stabilization of the polypropylene/polystyrene nanoblends prepared by diffusion and polymerization of styrene in isotactic polypropylene pellets during melt mixing by the incorporation of divinylbenzene. J. Appl. Polym. Sci. 127 (2013) 1092–1097. DOI:10.1002/app.v127.2 |
| [13] | H.Y. Qiu, F. Chen, Z.X. Guo, J. Yu. Preparation of polypropylene/poly(styrene-co-(butyl methacrylate)) nanoblends by diffusion and subsequent copolymerization of monomers in isotactic polypropylene pellets. Chin. J. Polym. Sci. 33 (2015) 1380–1388. DOI:10.1007/s10118-015-1686-z |
| [14] | G.H. Hu, H. Cartier, C. Plummer. Reactive extrusion:toward nanoblends. Macromolecules 32 (1999) 4713–4718. DOI:10.1021/ma981924y |
| [15] | S.H. Chan, Y.Y. Lin, C. Ting. Nanoblends of incompatible polymers by direct spaceconfined polymerization. Macromolecules 36 (2003) 8910–8912. DOI:10.1021/ma035174q |
| [16] | H. Shimizu, Y.J. Li, A. Kaito, H. Sano. Formation of nanostructured PVDF/PA11 blends using high-shear processing. Macromolecules 38 (2005) 7880–7883. DOI:10.1021/ma051395f |
| [17] | Y. Tao, J. Kim, J.M. Torkelson. Achievement of quasi-nanostructured polymer blends by solid-state shear pulverization and compatibilization by gradient copolymer addition. Polymer 47 (2006) 6773–6781. DOI:10.1016/j.polymer.2006.07.041 |
| [18] | Y.J. Li, Y. IWakura, L. Zhao, H. Shimizu. Nanostructured poly(vinylidene fluoride) materials by melt blending with several percent of acrylic rubber. Macromolecules 41 (2008) 3120–3124. DOI:10.1021/ma7027402 |
| [19] | A. Walther, K. Matussek, A.H.E. Mü ller. Engineering nanostructured polymer blends with controlled nanoparticle location using Janus particles. ACS Nano 2 (2008) 1167–1178. DOI:10.1021/nn800108y |
| [20] | R. Zhu, T. Hoshi, Y. Chishima, et al. Microstructure and mechanical properties of polypropylene/poly(methyl methacrylate) nanocomposite prepared using supercritical carbon dioxide. Macromolecules 44 (2011) 6103–6112. DOI:10.1021/ma2001965 |
| [21] | A. Kausar, S. Zulfiqar, M.I. Sarwar. Self-assembled nanoblends of functional polystyrene and a reactive aramid:morphological and thermomechanical profile, J. Appl. Polym. Sci., 2014, 131:. J. Appl. Polym. Sci. 121 (2014) . |
 2016, Vol. 27
2016, Vol. 27 


