b Advanced Materials Research Unit, Faculty of Science, King Mongkut's Institute of Technology Ladkrabang, Bangkok 10520, Thailand
Mercury (II) is one of the most harmful elements for mammalian bodies. The toxicity of mercury (II) can cause several health problems such as damage to the nervous system, kidneys and brain [1]. The determination of toxic mercury (II) in environmental and biological fluids mainly relies on atomic fluorescence spectroscopy [2], atomic absorption/emission spectroscopy [3], electrochemical spectroscopy [4] and inductive coupled plasma mass spectrometry [5]. Although, all of these techniques provide excellent sensitivity and selectivity, they are rather expensive instruments, tests are time-consuming, have high operating costs and there is some difficulty in the preparation of samples [6]. Recently, the advancement of nanomaterials has received great research interest because of their unique optical properties. The optical properties of nanomaterials depend on size, shape and geometry. They are well-known for their characteristic colors due to the surface plasmon resonance (SPR) [7]. Among nanomaterials, silver nanoparticles (AgNPs) have been widely applied in optical sensors or colorimetric sensors due to their intriguing electronic, chemical and optical properties [8]. AgNPs exploit the phenomena of SPR which is endowed with unique light scattering and absorption properties [9]. The SPR absorption band of AgNPs is found to be more sensitive to changes due to the fluctuations in the dielectric surrounding medium ofnanoparticles such as inter-particle distances, solvent and adsorbents [10]. For example, previously our group developed a colorimetric sensor of AgNPs/polyaniline composite multilayer thin films for ammonia sensing [11]. The strong color shift of multilayer thin films from orange-red to yellow was visible when exposed to an ammonia solution. Farhadi et al. [12] presented a sensitive SPR for mercury (II) based on an AgNPs solution. The SPR shifts are linearly related to mercury (II) concentration. The color of AgNPs could be directly observed by the naked eye, resulting in inter-particle distance, aggregation and decomposed state in the solution.Jarujamrus etal. [13] reported a selective unmodified AgNPs probe solution for colorimetric sensing of mercury (II). The unmodified AgNPs probe was found to display color shifts from yellow to light yellow with increasing mercury (II) concentration. The color changes could be ascribed to the oxidation of AgNPs by mercury (II). Upon the addition of mercury (II) with increasing concentration, the SPR progressively decreased, which shows a slight shift of λmax to the blue wavelength.
Although many AgNPs have been used widely in optical or colorimetric sensors for mercury (II) detection [14-17], no study has been published for mercury (II) colorimetric sensor based on a layer-by-layer (LbL) assembly of silver triangular nanoplates (AgTNPs). For this reason the objective was to develop a sensor, which could function in liquid mercury (II) using AgTNPs multilayer thin films. For the fabrication of the sensor, AgTNPs were prepared and assembled into multilayer thin films using a LbL deposition technique. Based on a simple electrostatic interaction between cationic poly(diallyldimethylammonium chloride) (PDADMAC) and anionic AgTNPs in the dipping step. The LbL deposition technique allows the fabrication of AgTNPs multilayer thin films and provides outstanding control over surface and thickness properties. AgTNPs were prepared using sodium citrate dihydrate as the stabilizing agent in order to deposit them into multilayer thin films. The resulting multilayer thin films were used as an optical sensor for mercury (II). The optical properties of AgTNPs multilayer thin films were characterized by a UV-visible spectroscopy. Surface morphology and thickness of multilayer thin films were evaluated by atomic force microscopy (AFM) and field emission scanning electron microscope (FESEM). The SPR spectra of AgTNPs sensing probe when exposed in different concentrations of mercury (II) were determined by UV-visible spectroscopy.
2. Experimental 2.1. MaterialsSilver nitrate (AgNO3), sodium borohydride (NaBH4), sodium citratedihydrate(C6H5Na3O7.2H2O), poly(diallyldimethylammo- nium chloride) (Mw 200, 000-350, 000gmol-1), poly(sodium 4- styrenesulphonate) (PSS, Average Mw 70, 000gmol-1), mercuric chloride (HgCl2) were purchased from Sigma-Aldrich Co., Ltd USA. Hydrogen peroxide (H2O2, 30 wt.%) was purchased from Carlo Erba Co., Ltd. All chemicals used were of analytical reagent grade (AR grade) and used without further purification. All solutions were prepared with double distilled water with a measured resistivity of 18.2 MVcm-1.
2.2. Synthesis of unmodified silver triangular nanoplatesUnmodified AgTNPs were prepared by the well-described method [18] using a chemical reduction process. For a typical synthesis, 50 mL of 1 mmol/L silver nitrate was mixed with 3 mL of 20mmol/L sodium citrate dihydrate. The solution was stirred for 5 min to get a homogeneous solution. Then 0.12 mL of 30 wt.% H2O2 was injected into the homogeneous solution. Freshly prepared NaBH4 (100 mmol/L, 0.33 mL) was rapidly added to the solution. The mixture was stirred for 30 min. The color of the mixture changed rapidly from light yellow to dark yellow. After that the colloidal solution turned to red, green and blue, respectively, indicating the formation of AgTNPs. The colloidal AgTNPs were stored at 4 ℃. The SPR characteristic of colloidal nanoparticles was measured by UV- visible spectroscopy. The size and morphology of nanoparticles were confirmed by transmission electron microscope (TEM).
2.3. Multilayer thin films assemblyThe multilayer thin films of AgTNPs were assembled onto a glass slide substrate. The cleaned glass slide substrate was immersed in 10 mmol/L PDADMAC for 5 min in order to generate a positively charged substrate. Then the substrate was rinsed three times with deionized water in order to remove the excess of PDADMAC. The glass slide substrates, which were positively charged, were immersed in a 10 mmol/L PSS solution for 5 min followed by rinsing three times in water. The electrostatic interaction between cationic PDADMAC and anionic PSS generated the hydrophilic glass slide substrates. In order to fabricate the AgTNPs multilayer thin films, the top layer of positively charged PDADMAC was dipped in an AgTNPs solution. The multilayer thin films of AgTNPs were achieved by repeating the number of layers with PDADMAC. The multilayer thin films were rinsed with deionized water and dried with air before being kept in a dark area.
2.4. Colorimetric detection of mercury (II)The prepared AgTNPs multilayer thin films were tested for their sensing properties in solution when exposed to solutions of different mercury (II) concentrations. The multilayer thin films of AgTNPs were then cut into 0.5 cm sections in order to fit in the cuvette and immersed in a 3 mL solution containing various concentrations of mercury(II) (0.5, 1, 5, 10, 15, 20, 25 and 30 ppm). The sensor was allowed to react for 5 min for the color of the sensor to be stable. Great care was taken to insure complete color change by recording UV-visible spectra until no changes in absorbance could be seen.
2.5. CharacterizationThe SPR of AgTNPs was measured in the 350-800 nm range by using a double beam UV1800, Shimadzu, China. This absorption wavelength was chosen because it covers the nanoparticles SPR located around 667 nm. A TEM model Jeol JEM 100SX was used to analyze the particle size and morphology of a fresh solution of AgTNPs. The surface charges and size distribution of AgTNPs were characterized using a NanoZS-Malvern instrument, UK. An atomic force microscopy model NanoScope IV-Veeco Instrument, USA, was used to study the surface topography and thickness of the multilayer thin films. AFM analysis was performed in air using a scanning probe microscope (SPM) multimode unit with a type RTESP silicon tip with a 125 μm length and 300 kHz resonance in tapping mode. X-ray diffractometer (XRD) was used to analyze the crystal structure of AgTNPs multilayer thin films (CuKa radiation, 2θ = 20-80°, Bruker D8 Advance, ). The FESEM (JSM-7001F, JEOL Solution for Innovation, Germany) was used to analyze the surface and morphology of AgTNPs multilayer thin films.
3. Results and discussionUnmodified AgTNPs were synthesized by chemical reduction of silver ions with NaBH4 by using sodium citrate as the stabilizing agent. H2O2 was used as the etchant for the triangular nanoplates formation [19]. The choice of sodium citrate for the stabilizing of AgTNPs was motivated by the chemistry of the substance which possesses the carboxylic functional groups. The carboxylic functional groups are well-known to adsorb and interact onto the surface of the nanoparticles [11]. These carboxylic functional groups are necessary to the design of the LbL system, which needs to target films assembled. UV-visible absorption spectra ofAgTNPs are shown in Fig. 1. The AgTNPs formed in the solution, through nucleation growth, displayed a characteristic vivid blue color (Fig. 1a), which is due to the surface plasmon absorbance phenomenon. The extinction spectrum exhibited a single peak around 730 nm. The size and morphology of AgTNPs were confirmed by TEM and their images are shown in Fig. 1b(1-2) revealing an average particle size of 35.14 ± 1.18nm (n =100) (Fig. 1c). The shape of nanoparticles appeared to be triangular nanoplates and no sign of aggregation was visible, confirming the good capping effect provided by sodium citrate. The zeta potential of AgTNPs stabilized with 20 mmol/L of sodium citrate was found to be -39.7 mV, which indicated the surface charge of sodium citrate stabilized nanoparticles.

|
Download:
|
| Figure 1. The UV-visible absorption spectra of AgTNPs. Insert (a) Digital image of AgTNPs solution, (b1-2) Typical TEM images ofAgTNPs at scale 100 and 50 nm and (c) Size distribution of AgTNPs | |
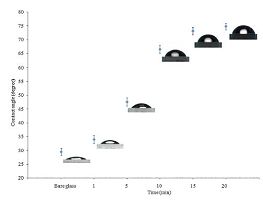
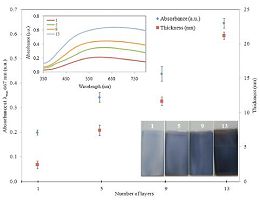
As mentioned earlier, the main point in using sodium citrate as the stabilizing agent for the preparation of AgTNPs is that they can be later assembled into a monolayer and later multilayer onto the substrate. The AgTNPs multilayer thin films were fabricated by alternate depositions of anionic sodium citrate stabilized AgTNPs and cationic PDADMAC. The main electrostatic interaction between quaternary ammonium groups of PDADMAC and carboxylic groups of sodium citrate stabilized AgTNPs is fundamental for the buildup of the multilayer thin films. Fig. 2 shows the kinetic absorption of the nanoparticles onto the glass slide substrate by plotting the contact angle of the monolayer of sodium citrate stabilized AgTNPs as the function of time. It can be seen from 1to 10 min the contact angle of the nanoparticles films increased sharply, which suggest quick adsorption of the nanoparticles onto the glass slide substrate, but stable when the deposition time was increased to15 min. As suggested in previous work [20], the increase in the contact angle of nanoparticles onto the substrate surface found more hydrophobic nanoparticles adsorbed in a more packed and dense fashion. The adsorption process is mainly controlled by two independent steps, which are the diffusion of the nanoparticles from the solution to the substrate and the complexation process. By taking advantage of electrostatic attraction between oppositely charged species, a film can be built with the desired thickness by controlling the number of dipping cycles. Fig. 3 shows the changes in thickness and absorbance as the function of the number of deposit layers of AgTNPs. The thickness and absorbance of multilayer thin films were characterized using AFM and UV-visible spectrophotometry, respectively. The multilayer thin films shown in the cartouche of Fig. 3 display a striking blue color due to the nanoparticles adsorption onto the surface of the substrate. The increase in thickness and absorbance are constant in each deposition step; probably due to the fact that the amount of nanoparticles is thermodynamically constant. The prepared multilayer thin films had a faster growth indicating an excellent bonding of the anionic sodium citrate stabilized AgTNPs and cationic PDADMAC.
|
Download:
|
| Figure 2. Plot of contact angle of the monolayer of AgTNPs as the function of time (Insert: Images of water droplets) | |
|
Download:
|
| Figure 3. The thickness and absorbance values as the function of number of layers of AgTNPs multilayer thin films and the representative digital images of AgTNPs multilayer thin films at 1, 5, 9 and 13 layers | |
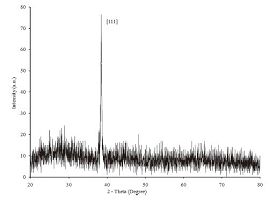
More evidence of the AgTNPs growth on the polyelectrolyte glass substrate can be seen from AFM images (Fig. 4). The surface morphology of the AgTNPs multilayer thin films is increasingly dense with individual AgTNPs when the number of layers is increased. The interconnection network between nanoparticle- nanoparticle had an impact on the root mean square (RMS) roughness values of the multilayer thin films. The RMS roughness values at 1, 5, 9 and 13 layers were found to increase from 3.923 ± 1.028, 5.486 ± 0.925, 9.840 ± 0.983 and 13.448 ± 0.847nm, respectively. In order to confirm the growth of AgTNPs onto the substrate surface, crystalline information of the nanoparticles was obtained by XRD measurement. The X-ray diffraction measurement (Fig. 5) indicated the prominent peaks at 2θ values of AgTNPs multilayer thin films at 38.4° corresponds to the reflection of the metallic silver particles crystallized in the FCC structure with basal [111] lattice plans. Based on the se observations, the crystalline structure of AgTNPs multilayer thin films was found to be in agreement with previous reports [21, 22], which confirm the LbL assembly of AgTNPs onto the glass slide substrate.

|
Download:
|
| Figure 4. Surface topography images and the RMS roughness of AgTNPs multilayer thin films as the function of number of layers | |
|
Download:
|
| Figure 5. XRD patterns of AgTNPs multilayer thin films | |
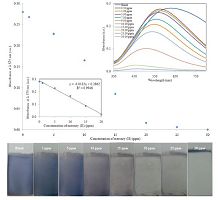
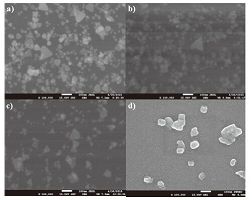
Using the LbL technique, the LbL technique, 5 layers of sodium citrate stabilized nd PDADMAC were sequentially deposited for evaluating ig efficiency toward mercury (II). In order to characterize ng properties of the films, the changes in SPR when :o increasing mercury (II) content (0, 0.5, 1, 5, 10, 15, 20, ppm) were monitored and plotted in Fig. 6. It can be seen characteristic blue peak centered at 667 nm gradually mauve peak centered at 570-452 nm as the mercury (II) s increased from 0.50 ppm to 25 ppm and appeared when the concentration of mercury (II) is increased to his color shift between blue to mauve and colorless was be very fast. The justification of such optical shift is due to the redox reaction between zero-valents of ind mercury (II). The AgTNPs deposited on glass slide were oxidized by mercury (II), resulting in the sition of AgTNPs into smaller particles and amalgam particles. This phenomenon was consisted with the studies [23-25]. For reference, the standard reduction of Hg2+/Hg and Ag+/Ag are 0.851 and 0.799 V, respec-tively [26]. In order to confirm this phenomenon, FESEM cuted to investigate the surface morphology of multilayer thin films before and after the addition of (II). The FESEM images shown in Fig. 7 were found the ation of AgTNPs particles into smaller particles and amalgam compound (Fig. 7b-d) when compared with the original AgTNPs multilayer thin films (Fig. 7a).
|
Download:
|
| Figure 6. UV–visible spectrum of AgTNPs multilayer thin films when exposed to various concentrations of mercury (II) (1, 5, 10, 15 20, 25 and 30 ppm) and the linearity for the detection of mercury (II) in the dynamic range of 0.5–20 ppm. Insert image is the photograph of AgTNPs multilayer thin films when exposed to various concentrations of mercury (II) | |
|
Download:
|
| Figure 7. FESEM images of (a) AgTNPs multilayer thin films and (b-d) are the FESEM images of AgTNPs multilayer thin films when exposed to mercury (II) concentrations from 1, 10 and 25 ppm | |
As shown in the cartouche of Fig. 6, a linear relationship between the concentration of mercury(II)and absorbance at λmax 579 was obtained in the range of 0.5-20 ppm, with a regression equation of y = -0.0132x + 0.2849 and a correlation coefficient (r2) of 0.9963. The limit of detection (LOD) at a signal to noise ratio of 3 and limit of quantitation (LOQ) at a signal to noise ratio of 10 were 0.45 ± 0.002 and 1.52 ± 0.002ppm, respectively. Comparison with other types of mercury (II) sensingprobe, the LOD reached by our approach is also much lower than 20 ppm for silver nanoliposome [27], 0.86 ppm for dichlorofluorescein-coumarin derivative [28] and 0.80 ppm for 1-pyrenecarboxaldehyde thiosemicarbazone [29] .
Although films prepared from 5 layers of sodium citrate stabilized AgTNPs and PDADMAC were sufficiently sensitive to be used as a mercury (II) sensor, the 7 and 9 layers displayed similar phenomenon with mercury (II) in the range of 0.5-20 ppm and the corresponding data is shown in Table 1. The similarity in linear range could be explained by thickness of AgTNPs multilayer thin films. The thickness of AgTNPs multilayer thin films at the number oflayers of 5, 7 and 9 were found in the range of7.414-11.638 nm. The inclusion of the thickness results serves to show that there is no significant difference between the thickness of AgTNPs multilayer thin films at the number oflayers of 5, 7 and 9. Thus, the number of mercury (II) that achieve contact with the sensing sites on the films were found constant and therefore induce similar detection of mercury (II) from the AgTNPs multilayer thin films.
|
|
Table 1 Comparison of the performance of different AgTNPs surface onto the mercury (II) sensing properties |
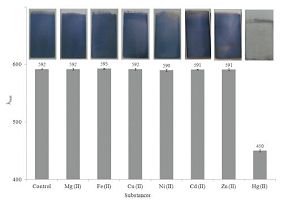
The selectivity of the AgTNPs multilayer thin films colorimetric sensor was evaluated through testing the response of assay toward the common cations such as Mg2+, Fe2+, Cu2+, Ni2+, Cd2+ and Zn2+ataconcentrationof20ppm.AsshowninFig. 8, the λmax of AgTNPs multilayer thin films when exposed to each of the common cations were lower by ± 2% than λmax of AgTNPs multilayer thin films, indicating that the AgTNPs multilayer thin films can selectively target for the detection of mercury (II). In order to evaluate the validity of our AgTNPs multilayer thin films colorimetric sensor toward mercury (II), two water samples were analyzed by spike different concentrations of mercury (II). As shown in Table 2, mercury(II) in concentrations of 5, 10and 20 ppm spiked in tap water and drinking water. The recovery values of AgTNPs multilayer thin films at three concentrations are in the range of 100.1%-106.4% for tap water and 100.2-104.0% for drinking water. Moreover the relative standard deviation (RSD) was found to be in the range of 0.4-1.6 and 0.4-2.2 for tap water and drinking water, respectively.
|
Download:
|
| Figure 8. The histogram of the colorimetric response of AgTNPs multilayer thin films to various cations | |
|
|
Table 2 Determination of mercury (II) in real water samples |
4. Conclusion
A LbL self-assembly technique was used to fabricate the multilayer thin films of unmodified AgTNPs. The deposition of the nanoparticles on glass slide substrate was controlled by the electrostatic interaction between cationic PDADMAC and anionic AgTNPs. The growth of the films was then pushed from the level of monolayer to the level of multilayer by alternating the dipping of the glass slide substrate in each cationic and anionic polyelectrolytes. A linear growth was observed for each dipping cycle. The optical sensing of AgTNPs multilayer thin films were tested against mercury (II) concentration. The color of AgTNPs multilayer thin films was observed from blue in the initial state to mauve and colorless when the concentration of mercury (II) increased from 0.5 to 30 ppm. The performance of the LbL assembly of unmodified AgTNPs multilayer thin films is attributed to high selectivity and sensitivity for mercury (II), which could be used as a colorimetric sensor for mercury (II) in aqueous environments.
AcknowledgmentThis work was supported by Faculty of Science, King Mongkut's Institute ofTechnology Ladkrabang, Bangkok, Thailand (No. 255901-05-033).
| [1] | Y. Wang, F. Yang, X.R. Yang. Colorimetric biosensing of mercury (II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens. Bioelectron. 25 (2010) 1994–1998. DOI:10.1016/j.bios.2010.01.014 |
| [2] | L. Beaudin, S.C. Johannessen, R.W. Macdonald. Coupling laser ablation and atomic fluorescence spectrophotometry:an example using mercury analysis of small sections of fish scales. Anal. Chem. 82 (2010) 8785–8788. DOI:10.1021/ac1021387 |
| [3] | N. Pourreza, K. Ghanemi. Determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry after solid phase extraction on agar modified with 2-mercaptobenzimidazole. J. Hazard Mater. 161 (2009) 982–987. DOI:10.1016/j.jhazmat.2008.04.043 |
| [4] | H.J. Kim, D.S. Park, M.H. Hyun, Y.B. Shim. Determination of HgII ion with a 1,11-bis(8-quinoyloxy)-3,6,9-trioxaundecane-modified glassy carbon electrode using spin-coating technique. Electroanalysis 10 (1998) 303–306. DOI:10.1002/(ISSN)1521-4109 |
| [5] | D. Karunasagar, J. Arunachalam, S. Gangadharan. Development of a ‘collect and punch’ cold vapour inductively coupled plasma mass spectrometric method for the direct determination of mercury at nanograms per litre levels. J. Anal. At. Spectrom. 13 (1998) 679–682. DOI:10.1039/A802132E |
| [6] | Z.B. Chen, T.H. Lou, Q. Wu, et al. A facile label-free colorimetric sensor for Hg2+ based on Hg-triangular silver nanoplates with amalgam-like structure. Sens. Actuators B:Chem. 221 (2015) 365–369. DOI:10.1016/j.snb.2015.06.082 |
| [7] | D. van Lierop, Ž. Krpetic, L. Guerrini, et al. Positively charged silver nanoparticles and their effect on surface-enhanced Raman scattering of dye-labelled oligonucleotides. Chem. Commun. 48 (2012) 8192–8194. DOI:10.1039/c2cc31731a |
| [8] | Y. Yang, S. Matsubara, L.M. Xiong, T. Hayakawa, M. Nogami. Solvothermal synthesis of multiple shapes of silver nanoparticles and their SERS properties. J. Phys. Chem. C 111 (2007) 9095–9104. DOI:10.1021/jp068859b |
| [9] | N. Chauhan, S. Gupta, N. Singh, et al. Aligned nanogold assisted one step sensing and removal of heavy metal ions. J. Colloid Interface Sci. 363 (2011) 42–50. DOI:10.1016/j.jcis.2011.07.018 |
| [10] | N. Bi, Y.H. Chen, H.B. Qi, et al. A sensitive localized surface plasmon resonance sensor for determining mercury (II) ion using noble metal nanoparticles as probe, Spectrochim. Acta A:. Mol. Biomol. Spectrosc. 95 (2012) 276–281. DOI:10.1016/j.saa.2012.04.086 |
| [11] | E. Detsri, J. Popanyasak. Fabrication of silver nanoparticles/polyaniline composite thin films using layer-by-layer self-assembly technique for ammonia sensing. Colloids Surf. A:Physicochem. Eng. Aspect. 467 (2015) 57–65. DOI:10.1016/j.colsurfa.2014.11.019 |
| [12] | K. Farhadia, M. Forougha, R. Molaeia, S. Hajizadeha, A. Rafipourb. Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sens. Actuators. B:Chem. 161 (2012) 880–885. DOI:10.1016/j.snb.2011.11.052 |
| [13] | P. Jarujamrus, M. Amatatongchai, A. Thima, T. Khongrangdee, C. Mongkontong. Selective colorimetric sensors based on the monitoring of an unmodified silver nanoparticles (AgNPs) reduction for a simple and rapid determination of mercury. Spectrochim. Acta A:Mol. Biomol. Spectrosc. 142 (2015) 86–93. DOI:10.1016/j.saa.2015.01.084 |
| [14] | L. Rastogi, R.B. Sashidhar, D. Karunssagar, J. Arunachalam. Gum kondagogu reduced/stabilized silver nanoparticles as direct colorimetric sensor for the sensitive detection of Hg2+ in aqueous system. Talanta 118 (2014) 111–117. DOI:10.1016/j.talanta.2013.10.012 |
| [15] | S.Y. Gao, X.X. Jia, Y.L. Chen. Old tree with new shoots:silver nanoparticles for label-free and colorimetric mercury ions detection. J. Nanopart. Res. 15 (2013) 1385. DOI:10.1007/s11051-012-1385-4 |
| [16] | G.L. Wang, X.Y. Zhu, H.J. Jiao, Y.M. Dong, Z.J. Li. Ultrasensitive and dual functional colorimetric sensors for mercury (II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens. Bioelectron. 31 (2012) 337–342. DOI:10.1016/j.bios.2011.10.041 |
| [17] | Y. Li, P. Wu, H. Xu, Z.P. Zhang, X.H. Zhong. Highly selective and sensitive visualizable detection of Hg2+ based on anti-aggregation of gold nanoparticles. Talanta 84 (2011) 508–512. DOI:10.1016/j.talanta.2011.01.037 |
| [18] | G.S. Mé traux, C.A. Mirkin. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater. 17 (2005) 412–425. DOI:10.1002/adma.v17:4 |
| [19] | J. Zhang, Y. Sun, H. Zhang, et al. Preparation and application of triangular silver nanoplates/chitosan composite in surface plasmon resonance biosensing. Anal. Chim. Acta 769 (2013) 114–120. DOI:10.1016/j.aca.2013.01.034 |
| [20] | N.A. Katov, L. Dekany, J.H. Fendler. Layer-by-layer self-assembly of polyelectrolyte-semiconductor nanoparticle composite films. J. Phys. Chem. 99 (1995) 13065–13069. DOI:10.1021/j100035a005 |
| [21] | P.K. Khanna, N. Singh, S. Charan, A.K. Viswanath. Synthesis of Ag/polyaniline nanocomposite via an in situ photo-redox mechanism. Mater. Chem. Phys. 92 (2005) 214–219. DOI:10.1016/j.matchemphys.2005.01.011 |
| [22] | S.K. Pillalamarri, F.D. Blum, A.T. Tokuhiro, M.F. Bertino. One-pot synthesis of polyaniline-metal nanocomposites. Chem. Mater. 17 (2005) 5941–5944. DOI:10.1021/cm050827y |
| [23] | T. Morris, H. Copeland, E. McLinden, S. Wilson, G. Szulczewski. The effects of mercury adsorption on the optical response of size-selected gold and silver nanoparticles. Langmuir 18 (2002) 7261–7264. DOI:10.1021/la020229n |
| [24] | A. Henglein, C. Brancewicz. Absorption spectra and reactions of colloidal bimetallic nanoparticles containing mercury. Chem. Mater. 9 (1997) 2164–2167. DOI:10.1021/cm970258x |
| [25] | L. Katsikas, M. Gutiérrez, A. Henglein. Bimetallic colloids:silver and mercury. J. Phys. Chem. 100 (1996) 11203–11206. DOI:10.1021/jp960357i |
| [26] | Y.J. Fan, Z. Liu, L. Wang, J.H. Zhan. Synthesis of starch-stabilized Ag nanoparticles and Hg2+ recognition in aqueous media. Nanoscale Res. Lett. 4 (2009) 1230–1235. DOI:10.1007/s11671-009-9387-6 |
| [27] | E. Priyadarshini, N. Pradhan, A.K. Pradhan, P. Pradhan. Label free and high specific detection of mercury ions based on silver nano-liposome. Spectrochim. Acta A:Mol. Biomol. Spectrosc. 163 (2016) 127–133. DOI:10.1016/j.saa.2016.03.040 |
| [28] | H.J. Kim, E.J. Park, M.G. Choi, S. Ahn, S.K. Chang. Selective chromogenic and fluorogenic signalling of Hg2+ ions using a fluorescein-coumarin conjugate. Dyes Pigments 84 (2010) 54–58. DOI:10.1016/j.dyepig.2009.06.009 |
| [29] | X.M. Wang, H. Yan, X.L. Feng, Y. Chen. 1-Pyrenecarboxaldehyde thiosemicarbazone:a novel fluorescent molecular sensor towards mercury (II) ion. Chin. Chem. Lett. 21 (2010) 1124–1128. DOI:10.1016/j.cclet.2010.04.029 |
 2016, Vol. 27
2016, Vol. 27