b School of Architecture and Construction, University of Science and Technology LiaoNing, Anshan 114051, China
Direct combustion of low rank coal and biomass leads to a lot of environmental problem and causes great concerns globally [1, 2]. More researches focused on comprehensive utilization of coal and agricultural residues recently [3-6]. The energy conversion technologies including co-pyrolysis, co-gasification and coliquefaction could use renewable resources, protect the environment, and increase energyefficiency. Some literatures investigated that low temperature pyrolysis was one of feasible methods to produce liquid fuels and high added-value chemicals [7-9]. Our previous study indicated the proper ratio of cotton stalk (CS) in blending of CS and Shenmu coal (SM) could improve the aliphatic hydrocarbon yield derived from co-pyrolysis tar of CS/SM, compared with that of SM pyrolysis tar [10]. Microcrystalline cellulose (MCC) was selected as a model compound to compare the difference effects of CS and cellulose about the yield and composition of aliphatic hydrocarbon in pyrolytic tar during copyrolysis. The reaction schemes of aliphatic hydrocarbon formation were inferred from the experimental results. The synthetic pathway of major products was proposed further.
2. Experimental 2.1. Raw materials and process of pyrolysisThe air-dried samples were milled and sieved to obtain fractions of particle size less than 180 μm in diameter for both SM and CS. The particle size of MCC (AR grade) was less than 60 mm and its degree of polymerization was 3000-10, 000. The pyrolysis experiments were carried out in a tubular furnace (from room temperature to 873 K at 5 K/min, then kept 15 min). The methods of yield calculation and product analyses are in identical manner with our previous report [10]. The cellulose content in CS was determined 45% ± 0.03 by the Van Soest method [11]. Therefore, the blending ratio of MCC/SM was 9/100 which matched the blending ratio of CS/SM (20/100) during copyrolysis.
2.2. GC/MS analyses of n-hexane soluble in the pyrolytic tarn-hexane (GR grade) was applied to extract non-polar and weak-polar compounds from pyrolysis tar. The compounds of n- hexane soluble were characterized by GC-MS (Agilent 5975C, USA). High-purity helium was chosen as the carrier gas. The yield of each compound was defined as the results of GC/MS analyses and the yield of n-hexane soluble in pyrolysis tar.
3. Results and discussion 3.1. Effects of CS in the formation of aliphatic hydrocarbon during copyrolysisThe categories of n-hexane soluble in pyrolysis tar are presented in Table 1. The aliphatic hydrocarbon yield of CS/SM co-pyrolysis tar is 45.45 wt% (tar) and 46.28% more than that of SM pyrolysis tar (31.07 wt%, tar). The results show that CS could improve the formation of aliphatic hydrocarbon during copyrolysis of CS/SM. Because the aliphatic hydrocarbon yield of MCC/SM co-pyrolysis tar (40.77 wt%, tar) is also higher than that of SM pyrolysis tar, it is shown cellulose could increase the content of aliphatic hydrocarbon in the co-pyrolysis tar. And because the aliphatic hydrocarbon yield of MCC/SM co-pyrolysis tar is less than that of CS/SM co-pyrolysis tar, other ingredients in CS such as lignin and hemicellulose might improve the formation of aliphatic hydrocarbons during co-pyrolysis as well.
|
|
Table 1 Categories of n-hexane soluble in pyrolysis tar (wt%, tar). |
As the shown in Table 2, the aliphatic hydrocarbons in the pyrolysis tar of SM, CS/SM and MCC/SM can be grouped in three categories: chain alkenes, branched alkenes and olefins. Moreover, cycloalkanes are not detected in their tar. While the aliphatic hydrocarbon yield of CS pyrolysis tar is 41.06 wt% (tar) (Table 1) and 72.39% of the aliphatic hydrocarbons are cyclohexane and methylcyclopentane resulted in the GC/MS analyses. The above observation indicated that there was an interaction between the volatile matters of SM and CS which accelerated the formation and stabilization of aliphatic hydrocarbons during co-pyrolysis.
|
|
Table 2 Categories of aliphatic hydrocarbons in pyrolysis tar (wt%, tar). |
The main aliphatic hydrocarbon products in SM pyrolysis tar are chain alkanes (24.02 wt%, tar) as list in Table 2. Its content is 77.32% in the aliphatic hydrocarbons. Compared with SM pyrolysis, CS/SM co-pyrolysis increased the yields of all kinds of aliphatic hydrocarbons, especially for olefins and branched alkanes. The yields of olefins and branched alkanes in co-pyrolysis tar of CS/SM are 12.24 wt% (tar) and 6.92 wt% (tar), respectively. The co-pyrolysis tar of MCC/SM has the minimum yield of branched alkanes (1.26 wt%, tar) in comparison with SM pyrolysis tar and co-pyrolysis tar of CS/SM. While the yields of chain alkanes and olefins in co-pyrolysis tar of MCC/SM are maximum than that of CS/SM co-pyrolysis tar and SM pyrolysis tar. Therefore, the CS addition could significantly increase the content of olefins and branched alkanes in pyrolysis tar during co-pyrolysis of CS/SM. Furthermore, the cellulose conduced particularly to the formation of olefins and chain alkanes in co-pyrolysis tar.
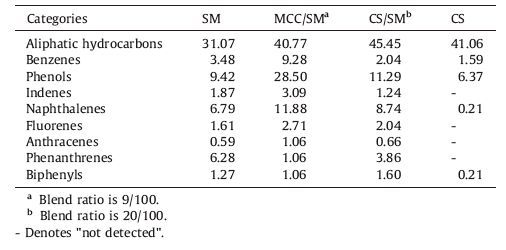
3.2. Generation of alkyl radicals during pyrolysisCo-pyrolysis of coal and biomass is well recognized as a radical mechanism [12-14]. With pyrolysis temperature increasing, large numbers of volatile radical fragments were produced due to the thermal cleavage of covalent bonds. Some of radicals coupled subsequently with each other to form stable aliphatic hydrocarbons along with the secondary pyrolysis. For the aliphatic hydrocarbons of co-pyrolysis tar were composed of chain alkanes, olefins and branched alkanes, the aliphatic hydrocarbons were mainly formed by the alkyl radical reactions. Fig. 1 is the generation of alkyl radicals from SM pyrolysis. The paraffin hydrocarbons in texture of coal were decomposed into alkyl radicals which were macromolecular radicals during SM pyrolysis. Other alkyl radicals of SM pyrolysis were generated from the cleavage of alkyl side-chains and bridge bonds cracking in aromatic structure of coal. This kind of radicals were mostly C1-C5 micromolecular radicals [15].

|
Download:
|
| Figure 1. Generation of alkyl radicals from SM pyrolysis. | |
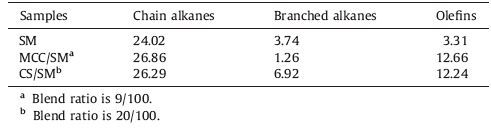
The alkyl radicals of CS pyrolysis are also micromolecular radicals and some have unsaturated group, as shown in Fig. 2. In this pathway, the alkane radicals are mainly derived from the side-chains cleavage and bridge bonds cracking of lignin. Meanwhile cellulose and hemicellulose are converted to furans by depolymerization and ring-opening. With the pyrolysis temperature increasing, the furans tended to crack because of its poor thermostability [16, 17]. Some furans are converted to levulinic acid by deoxidation, which is then converted to pentenoic acid. The pentenoic acid is further converted to olefin radicals by decarbonylation. Other furans are converted to the olefin radicals with two unpaired electrons through losing the oxygen in heterocycle. The cellulose and hemicellulose can also be converted to acetaldehyde, which is further converted to C2 alkane radical by deoxidation [18-20].
|
Download:
|
| Figure 2. Generation of alkyl radicals from CS pyrolysis. | |
3.3. Synthetic pathway of aliphatic hydrocarbon during co-pyrolysis
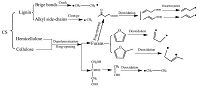
Based on the results of GC/MS analyses and the generative process of alkyl radicals, the possible synthetic pathways of aliphatic hydrocarbons were discussed as follow during copyrolysis. Chain alkane formation was due to the alkyl radicals which generated from the decomposition of SM and the lignin in CS. The radical reactivity has a great effect on synthetic pathways of aliphatic hydrocarbons. Macromolecular radicals have lower reactivity than micromolecular radicals. Because of two unpaired electrons in both ends, the radicals derived from bridged bond cracking have highly reactive towards that from cleavage of side- chain. As shown in Fig. 3a and b, macromolecular alkane radical combines with micromolecular alkane radicals to form chain alkane. The chain alkanes can also generated from micromolecular alkane radicals by coupling reactions as seen in Fig. 3c and d. Because the chain alkane yield of SM pyrolysis is lower than that of MCC/SM co-pyrolysis tar and that of CS/SM co-pyrolysis tar (list in Table 2), it was determined that the alkyl radicals from CS could improve the formation of chain alkanes. The GC/MS results of pyrolysis tar showed that CS could increase the content of C12-C25 chain alkanes (except for C19) in the co-pyrolysis tar of CS/SM.

|
Download:
|
| Figure 3. Synthetic pathway of chain alkane formation during co-pyrolysis. | |
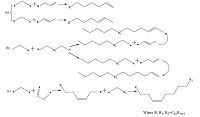
The olefins were formed by the radical polyreaction during copyrolysis of CS/SM. Compared with the alkane radicals in same amount of carbon atoms, olefin radicals had more reactivity due to asymmetric structure and double bond. As shown in Fig. 4a and b, the kind of monoolefines are generated from the coupling of olefin radicals and alkane radicals by chain reactions, such as 1-undecene and 2-tetradecene, (E)-. The olefin radical of two unpaired electrons can copolymerize with alkane radicals to produce the internal-olefin such as 9-tricosene, (Z)-. The possible pathway to produce the internal-olefin is shown in Fig. 4c. Moreover, this kind of olefin radicals which had two unpaired electrons could also couple with non-alkyl radicals to form polar compounds. The double bond was opened by electrophilic addition easily. Therefore, the internal-olefin content in pyrolysis tar was extremely low. The yields of the internal-olefin in copyrolysis tar of CS/SM and MCC/SM were only 1.54 wt% (tar) and 1.33 wt% (tar), respectively. And the internal-olefin yield was 1.15 wt% (tar) in SM pyrolysis tar. The above observation indicated that olefins in co-pyrolysis tar were formed from two approaches. One was the addition reaction of radicals from SM and the cellulose in CS during co-pyrolysis. The other one was derived from SM pyrolysis alone.
|
Download:
|
| Figure 4. Synthetic pathway of olefin formation during co-pyrolysis. | |
Olefins could convert to branched alkane through radical addition reactions, which occur by opening the double bond. Compared with the branched alkane yield in co-pyrolysis tar of CS/ SM (6.92 wt%, tar), that yield of MCC/SM co-pyrolysis tar was only 1.26 wt% (tar) because of the lack of alkyl radicals generated from lignin and hemicellulose. The branched alkane yield of SM pyrolysis was 3.74 wt% (tar) and lower than that of CS/SM copyrolysis. It was indicated that the olefins would be converted to branched alkanes by radical addition resulting in increasing the content of branched alkane in co-pyrolysis tar of CS/SM. Polyene was converted more easily than monoolefine due to its polyunsaturated asymmetric structure. The synthetic pathway is seen in Fig. 5a and b. Undecane, 2, 6-dimethyl- is formed as the synthetic pathway of Fig. 5b, 0.33% of CS/SM co-pyrolysis tar. The polyene can also be generated from olefin radicals by codimerization, which is further converted to branched alkane by radical additions as shown in Fig. 5c. The content of decane, 2, 3, 6-trimethyl- in copyrolysis tar of CS/SM was 0.73%. The yield of poly-substituted branched alkanes (4.08 wt%, tar) was higher than the yield of mono-substituted branched alkanes (0.95 wt%, tar) in co-pyrolysis tar of CS/SM. MCC/SM co-pyrolysis tar had 1.06 wt% (tar) poly- substituted branched alkanes and was not detected the mono- substituted branched alkanes. It was decided further that polyene had more reactivity than monoolefine. Additionally, the results showed that olefins had inhibition to convert to branched alkanes in lacking of alky radicals.

|
Download:
|
| Figure 5. Synthetic pathway of branched alkane formation during co-pyrolysis. | |
4. Conclusion
Low temperature pyrolysis were carried out in a tubular furnace. The aliphatic hydrocarbon yield was 45.45 wt% in copyrolysis tar of CS/SM and mainly formed from alkyl radicals by radical reactions. The chain alkanes were generated from alkane radicals by combination and coupling. And the experimental results indicated that the alkyl radicals derived from CS could improve the yield of C12-C25 chain alkanes during co-pyrolysis of CS/SM. Compared with SM pyrolysis tar, the aliphatic hydrocarbon yield of CS/SM co-pyrolysis tar increased obviously due to amount of alkyl radicals derived from CS, especially for the yields of olefins and branched alkanes. The olefin radical combined with alkyl radicals to form olefins by radical additions. The pyrolysis of cellulose and hemicellulose in CS produced mostly olefin radicals due to promote significantly the olefin yield of co-pyrolysis tar. The alkyl radicals from CS also would enhance the isomerization of olefins. Polyene and some of monoolefine were further converted to branched alkanes by radical additions during co-pyrolysis of CS/SM.
AcknowledgmentThe work was supported by the National Basic Research Program of China (973 program, No. 2011CB201304).
| [1] | M. Wang, M. Shao, S.H. Lu, Y.D. Yang, W.T. Chen. Evidence of coal combustion contribution to ambient VOCs during winter in Beijing. Chin. Chem. Lett. 24 (2013) 829–832. DOI:10.1016/j.cclet.2013.05.029 |
| [2] | L.Y. Li, Y. Chen, L.M. Zeng, et al. Biomass burning contribution to ambient volatile organic compounds (VOCs) in the Chengdu-Chongqing Region (CCR), China. Atmos. Environ. 99 (2014) 403–410. DOI:10.1016/j.atmosenv.2014.09.067 |
| [3] | F.M. Lin, E.N.G. Mash, X.N. Lin. Recent progress in hydrocarbon biofuel synthesis:pathways and enzymes. Chin. Chem. Lett. 26 (2015) 431–434. DOI:10.1016/j.cclet.2015.03.018 |
| [4] | P. Liu, L.L. Wang, Y. Zhou, et al. Effect of hydrothermal treatment on the structure and pyrolysis product distribution of Xiaolongtan lignite. Fuel 164 (2016) 110–118. DOI:10.1016/j.fuel.2015.09.092 |
| [5] | J.F. Wang, J.T. Zhao, F.H. Li, et al. Product characteristics for fast co-pyrolysis of bituminous coal and biomass. J. Fuel Chem. Technol. 43 (2015) 641–648. |
| [6] | S.D. Li, X.L. Chen, G.S. Yu, F.C. Wang. Study on co-pyrolysis behavior and model of biomass and coal. Acta Energiae Solaris Sin. 35 (2014) 965–970. |
| [7] | C. Dong, L.J. Jin, S.J. Tao, Y. Li, H.Q. Hu. Xilinguole lignite pyrolysis under methane with or without Ni/Al2O3 as catalyst. Fuel Process. Technol. 136 (2015) 112–117. DOI:10.1016/j.fuproc.2014.10.037 |
| [8] | P.F. Wang, L.J. Jin, J.H. Liu, S.W. Zhu, H.Q. Hu. Analysis of coal tar derived from pyrolysis at different atmospheres. Fuel 104 (2013) 14–21. DOI:10.1016/j.fuel.2010.06.041 |
| [9] | F.X. Collard, J. Blin. A review on pyrolysis of biomass constituents:mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 38 (2014) 594–608. DOI:10.1016/j.rser.2014.06.013 |
| [10] | C.Y. Tang, D.X. Zhang, X.L. Lu. Improving the yield and quality of tar during copyrolysis of coal and cotton stalk. Bioresources 10 (2015) 7667–7680. |
| [11] | P.J. Van Soest, R.H. Wine. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant-cell constituents. J. Assoc. Off. Anal. Chem. 50 (1967) 50–55. |
| [12] | D.X. Zhang, S.C. Wang, X.L. Ma, Y.Q. Tian. Interaction between coal and distillation residues of coal tar during co-pyrolysis. Fuel Process. Technol. 138 (2015) 221–227. DOI:10.1016/j.fuproc.2015.06.002 |
| [13] | W.J. He, Q.Y. Liu, L. Shi, et al. Understanding the stability of pyrolysis tars from biomass in a view point of free radicals. Bioresour. Technol. 156 (2014) 372–375. DOI:10.1016/j.biortech.2014.01.063 |
| [14] | D.X. Zhang, P. Liu, X.L. Lu, L.L. Wang, T.Y. Pan. Upgrading of low rank coal by hydrothermal treatment:coal tar yield during pyrolysis. Fuel Process. Technol. 141 (2016) 117–122. DOI:10.1016/j.fuproc.2015.06.037 |
| [15] | P. Liu, D.X. Zhang, L.L. Wang, et al. The structure and pyrolysis product distribution of lignite from different sedimentary environment. Appl. Energy 163 (2016) 254–262. DOI:10.1016/j.apenergy.2015.10.166 |
| [16] | C.Q. Dong, Z.F. Zhang, Q. Lu, Y.P. Yang. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood. Energy Convers. Manag. 57 (2012) 49–59. DOI:10.1016/j.enconman.2011.12.012 |
| [17] | C.B. Lu, J.Z. Yao, W.G. Lin, W.L. Song. Study on biomass catalytic pyrolysis for production of bio-gasoline by on-line FTIR. Chin. Chem. Lett. 18 (2007) 445–448. DOI:10.1016/j.cclet.2007.01.005 |
| [18] | S. Ogo, T. Nishio, H. Sekine, A. Onda, Y. Sekine. One pot direct catalytic conversion of cellulose to C3 and C4 hydrocarbons using Pt/H-USY zeolite catalyst at low temperature. Fuel Process. Technol. 141 (2016) 123–129. DOI:10.1016/j.fuproc.2015.06.032 |
| [19] | H.Q. Sui, P. Li, X.H. Wang, et al. Influence on bio-oil by fractional condensation of biomass pyrolysis vapor. CIESC J. 66 (2015) 4138–4144. |
| [20] | X.X. Zhang, G.Y. Chen, W.C. Ma, B.B. Yan, Y.B. Li. Experimental research on copyrolysis of corn-cob and waste oil in a fixed-bed reactor. Acta Energ. Solaris Sin. 36 (2015) 2059–2064. |
 2016, Vol. 27
2016, Vol. 27