Owing to the tunable emitting properties and the flexibility for chemical modifications, phosphorescent iridium complexes have been intensively studied as emitting dopants in organic light emitting diodes (OLEDs) and light emitting electrochemical cells (LECs) [1-5]. In addition, certain iridium complexes were also found to be applied in phosphorescence imaging in live cells [6-12]. Compared with the fluorescent organic dyes, phosphorescent iridium complexes display the advantages such as large Stokes shift, fine photostability, high internal quantum yield (nearly 100% in common organic solvent due to triplet harvesting effect). Moreover, their long lifetime is helpful to eliminate the interference from autofluorescence of cells and tissues [13]. Moreover, the distorted octahedral structure of Ir(III) complexes is helpful to avoid the aggregation-caused quenching (ACQ) effect that is commonly observed for the planar organic fluorophores [14]. Although some Ir(III) phosphorescent complexes have been reported as imaging agents, most of them, such as [(ppy)2Ir(bpy)]+(PF6)- (ppy = 2-phenylprydine, bpy = 2, 2′-bipyridyl, PF6 = hexafluorophosphate) show relative low quantum yield due to their cationic nature [15]. On the other hand, the neutral iridium phosphors with high quantum yield, such as fac-Ir(ppy)3, FIrpic (bis[(4, 6-difluorophenyl)-pyridinate-N, C2]-picolinate) and (piq)2Ir(acac) (piq = 1-phenylisoquinoline, acac = acetylacetonate), usually have low water affinity, which disfavor the cell staining. Exploring Ir(III) complexes bearing both the high quantum yield and fine aqueous solubility is challenging and helpful for their application in cell imaging.
It was reported that the homoleptic Ir(III) complexes being modified with the hydrophilic amino acid shows the improved aqueous solubility [16], yet the modification of the ligand results in the tedious preparation procedure of these Ir(III) complexes. Considering amino acids (AA) are fine chelator with carboxyl and amino group as metal coordination groups, amino acid might serve as ancillary ligand directly to form the Ir(ppy)2(AA) complex. Therefore, both the molecular volume and charge can be reduced, and the high internal quantum efficiencies can be expected. In this work, two neutral Ir(III) complexes, (dfppy)2Ir(L-alanine) (dfppy = 2-(2, 4-difluorophenyl)pyridine) and (piq)2Ir(L-alanine) were synthesized readily with L-alanine as ancillary ligand (Scheme 1). Due to the fine biocompatibility and water solubility of the L-alanine ligand, these new Ir(III) complexes were applied practically in cell imaging as the phosphorescent agent.
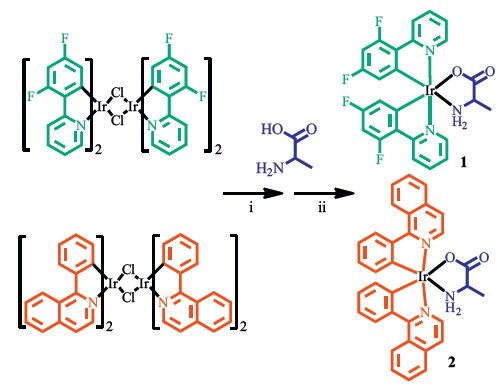
|
Download:
|
| Scheme. 1. Chemical structures and the general synthetic scheme of compounds 1 and 2. (i) t-BuOK, MeOH, room temperature; (ii) iridium µ-Cl dimer complexes, 2- ethoxylethanol, 120 ℃. | |
2. Experimental 2.1. General information
All reactions were carried out under nitrogen atmosphere. An electrospray ionization (ESI) mass spectrometer (LCQ fleet, Thermo Fisher Scientific) was used to record mass spectra and highresolution mass spectra were measured with an Agilent 6540 UHD Accurate-Mass Q-TOF LC/MS. 1H NMR spectra were measured on a Bruker AM 300 spectrometer. UV-vis absorption and photoluminescence spectra were obtained using a Shimadzu UV-31″ and a Hitachi F-46″ spectrophotometer respectively.
2.2. SynthesesThe general synthetic procedure for these complexes was shown in Scheme 1. Main ligands dfppy (2-(2, 4-difluorophenyl)- pyridine) and piq were adopted for their ability to endow the Ir(III) complexes with greenish-blue and red emission [17-19]. The cyclometallated main ligands dfppy and piq were synthesized with modified Suzuki cross coupling and the intermediate iridium µ-Cl dimer complexes were obtained directly by reacting main ligands with iridium chloride according to the reported methods [2-6]. LAlanine (2.4 µmol) was dissolved in 10 mL methanol and 5 mL methanol solution of t-BuOK (2.4 µmol) was added to the amino acid solution dropwise at room temperature for 2 h. Then the methanol solution of L-alanine potassium salt was added to a stirred solution of 1 µmol Ir(III) dimer in 20 mL 2-eyhoxyethanol at 120 ℃ dropwise and the reaction was kept at 120 ℃ for 12 h. Later, the solvent was removed in vacuum and the crude product was extracted with water and dichloromethane. The organic layer was collected and condensed. Rapid chromatography (silicon, eluent:ethyl acetate/hexanε = 1/2 v/v) and recrystallization (methanol/ dichloromethane) were performed to obtain pure product (dfppy)2Ir(L-alanine) and (piq)2Ir(L-alanine).
(dfppy)2Ir(L-alanine): Yellow powder, 0.62 g, yield 47%. 1H NMR (500 MHz, DMSO): δ 9.15 (dd, 1H, J = 44.7, 5.5 Hz), 8.64 (dd, 1H, J = 18.4, 5.8 Hz), 8.26 (d, 2H, J = 7.8 Hz), 8.20-8.04 (m, 2H), 7.76- 7.41 (m, 2H), 6.71 (dt, 2H, J = 19.9, 9.4 Hz), 5.85-5.67 (m, 1H), 5.51-5.31 (m, 1H), 2.51 (s, 4H). ESI-MS calcd.: m/z 662.1 for [M+H]+ (C25H19F4IrN3O2 +), foundm/z 662.1. High-resolution EI-MS calcd.: m/z 662.1037 for [M+H]+ (C25H19F4IrN3O2 +), found m/z 662.1036.
(piq)2Ir(L-alanine): Red powder, 0.70 g, yield 51%. 1H NMR (300 MHz, CDCl3): δ 8.94 (t, 2H, J = 19.6 Hz), 8.78 (d, 1H, J = 12.9 Hz), 8.45 (d, 1H, J = 13.2 Hz), 8.26-8.05 (m, 2H), 8.00 (d, 1H, J = 5.4 Hz), 7.95-7.84 (m, 1H), 7.83-7.62 (m, 5H), 7.61-7.37 (m, 2H), 6.87 (t, 2H, J = 11.9 Hz), 6.71-6.42 (m, 3H), 6.15 (d, 1H, J = 8.4 Hz), 1.79 (s, 3H). ESI-MS calcd.: m/z 690.2 for [M+H]+ (C33H27IrN3O2 +), found m/z 690.4. High-resolution EI-MS calcd.: m/z 690.1727 for [M+H]+ (C33H27IrN3O2 +), found m/z 690.1725.
2.3. Theoretical calculationsThe density functional theory calculations were carried out using Gaussian 09 software package. The basis set of LANL2DZ and 6-31G** were chosen for iridium atom and non-iridium atoms respectively. The geometries of the ground state were fully optimized with the B3LPY exchange-correlation functional both in vacuum and in DCM solution. Solvent effect was taken into consideration by using C-PCM solvent model. Vibrational frequency calculations were performed to validate that the optimized structures were minima on potential energy surface.
2.4. Cell imaging experimentHuman gastric cancer SGC-7901 cells were cultured in glass bottom dishes and seeded at a density of 1 - 106 cells/mL in RPMI 1640 supplemented with 10% FBS, NaHCO3 (2 g/L) and 1% antibiotics (penicillin/streptomycin, 100 U/mL) containing 10 µmol/L iridium probes (in DMSO/culture medium, 1/99, v/v) for 60 min at 37 ℃. One day before imaging, cells were passed and plated on 18-mm glass bottom dishes. Cell imaging was carried out after washing cells with PBS for three times. Confocal fluorescence imaging studies were performed with a ZEISS Laser Scanning Microscope (Zeiss LSM 710). The cell-imaging was investigated under 405 nm excitation.
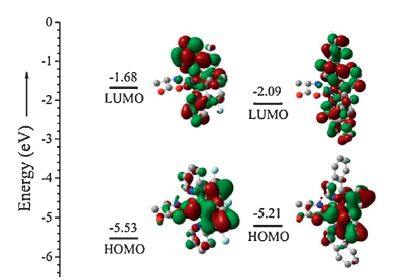
3. Results and discussionSince amino acid is not the conventional ancillary ligand for iridium phosphor complex, DFT calculations were carried out to gain more reasonable insight of the electronic structures [20]. From the calculation results (Fig. 1) we found that the HOMOs of the complexes are mainly consisted of p orbitals of phenyl rings in cyclometallated ligands and iridiumatom, the LUMOs are located on both phenyl and pyridine rings. Similar with widely used ancillary ligands like acac and tpip (tetraphenylimidodiphosphinate), amino acid contribute little to both HOMOs and LUMOs [21-23]. This demonstrates that the photophysical properties of the Ir(III) complexes with amino acid ancillary ligands, such as emission color and phosphorescent lifetime are determined mainly by the main ligands. This implies the possibility to predict the phosphorescence of this kind of iridiumcomplexes with amino acid ancillary ligands, favoring for their imaging application.
|
Download:
|
| Figure 1. Frontier molecular orbitals of complexes 1 and 2 (B3LYP/6-31G**, LANL2DZ). | |
In order to investigate the photophysical properties of the two complexes, the absorption and emission spectra were recorded in dichloromethane (Fig. 2, Table S1 in Supporting information). As illustrated in Fig. 2a, both (dfppy)2Ir(L-alanine) and (piq)2Ir(L-alanine) have strong inter-ligand π-π* charge transfer (ILCT) absorptions in the wavelength range of 250- 330 nm with maximum extinction coefficients larger than 35 000 L mol-1 cm-1. The absorption bands in the range of 330-430 nm are generated by spin allowed metal to ligand charge transfer (1MLCT) absorption overlapped by the tail of ILCT. In the range longer than 430 nm, there are still weak absorptions for both complexes and the intensity are similar. These can be assigned as the forbidden 3MLCT absorption due to the strong spin-orbital coupling effect of iridium center. In Fig. 2b, complexes (dfppy)2Ir(L-alanine) and (piq)2Ir(L-alanine) show broad emission bands with full width at half maxima (FWHM) of 56 nm and 73 nm, respectively. Their emission peaks locate at 477 and 622 nm with high quantum yields (62.0% and 60.6%) at room temperature. At 77 K, their emission bands are divided into two peaks and the main peaks have a hyposochromatic shift of 5 nm and 21 nm, respectively, demonstrating the hybrid emission state of 3MLCT and 3LC characters [24]. In comparisonwith most organic fluorescent dyes, phosphorescent complexes (dfppy)2Ir(L-alanine) and (piq)2Ir(L-alanine) display the large Stokes shift and the complete separation of emission band from MLCT absorption band, which is helpful to reduce the self-absorption effect in cell imaging.

|
Download:
|
| Figure 2. UV-vis absorption (a) and emission (b, normalized) spectra of complexes 1 and 2 in dichloromethane (1 - 10-5 mol/L). Inset: the emission spectra of complexes 1 and 2 (5 - 10-5 mol/L) in water containing 1% DMSO (v/v) at 298 K. | |
The emission spectra of (dfppy)2Ir(L-alanine) and (piq)2Ir(Lalanine) in 1% DMSO aqueous solutions were also shown in Fig. 2 (inset). Both complexes display the emission spectra similar to that shown in organic solvent without obvious shifts and widening. Furthermore, the emission spectra in different acidic aqueous solutions were also measured (Fig. S1 in Supporting information). With the change of pH value, the emission figure and intensity have slight difference, indicating that the photophysical properties of these two iridium complexes are stable to the environment. However, compared with those in neutral solution, the emission peaks of these two complexes present redshifts of around 20 nm and 30 nm respectively, might be caused by the hydrogen bond interaction on carboxyl group. The fine aqueous solubility and high quantum yield suggest the two complexes might be suitable candidate as cell imaging agent.
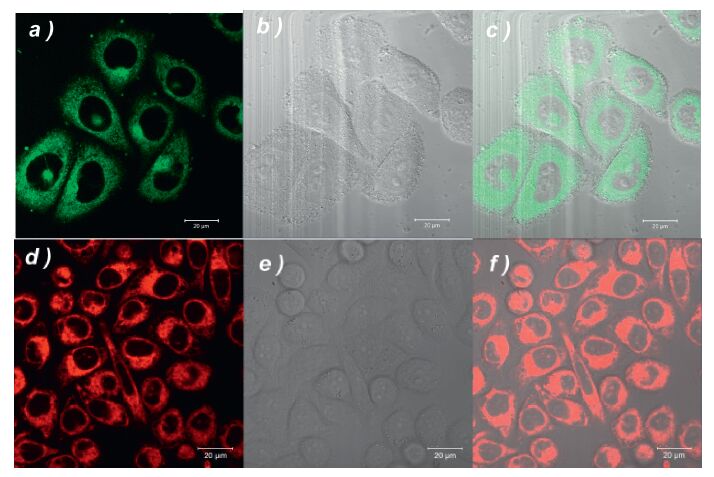
The imaging experiments were investigated in human gastric cancer SGC-7901 cells. As shown in Fig. 3, the distinct intracellular phosphorescence observed after 60 min of incubation with complex solution (10 µmol/L) at 25 ℃ for (λex: 405 nm; band path: 600-740 nm) indicates the fine cell membrane permeability of the two complexes. The superimposed images demonstrate that (dfppy)2Ir(L-alanine) and (piq)2Ir(L-alanine) are internalized in the cells rather than merely coloring the membrane. It is noted that both molecules are precisely localized in the cytoplasm and almost excluded from the nucleus. This result demonstrates that these two complexes are potential tools for cytoplasm visualization. In addition, they are possible to act as labeling tool to show the transportation of bioactive component into cytoplasm and the related cytoplasmic processes, such as translation of mRNA, vesicular trafficking and glycolysis, can be visualized. The current results suggested that the introduction of amino acid as ancillary ligands would lead to the neutral Ir(III) complexes, which can be utilized in aqueous cell culture media and intracellular microenvironment. Moreover, both complexes show low cell toxicity (Fig. S2 in Supporting information). According to the IC50 data, complex 1 (IC50 = 13.61 ≤ 0.36 µmol/L) presents lower cell toxicity than 2 (9.62 ≤ 0.02 µmol/L).
|
Download:
|
| Figure 3. Confocal microscopy image of SGC-7901 human gastric cancer cells treated with complexes 1 (top line) and 2 (bottom line). Left panel: fluorescence images, middle panel: bright field images and right panel: superimposed images. Cells were seeded at a density of 1 - 106 cells/mL in RPMI 1640 supplemented with 10% FBS, NaHCO3 (2 g/L) and 1% antibiotics (penicillin/streptomycin, 100 U/mL). The cells were maintained in a humidified incubator at 37 ℃, in 5% CO2/95% air. Cell imaging was carried out after washing cells with PBS for three times. Confocal fluorescence imaging studies were performed with a ZEISS Laser Scanning Microscope (Zeiss LSM 710). | |
4. Conclusion
In conclusion, we have constructed two neutral iridium phosphorescent complexes ((dfppy)2Ir(L-alanine) and (piq)2Ir(Lalanine)) with ppy and piq as themain ligands and one amino acid (L-alanine) as ancillary ligand. The two complexes demonstrate bright greenish-blue and red emission respectively, and their improved aqueous solubility and the retained quantum yield favor their application in cell imaging. Theoretic study demonstrated that the emission nature of these complexes is mainly determined by the main ligand. Intracellular imaging suggested that these two complexes have fine cell membrane permeability and is mainly distributed in cytoplasm. This study displayed a new strategy to design aqueous soluble phosphorescent cyclometallated Ir(III) complex via introducing amino acid as ancillary ligand, which also give promising clues to design responsive probe in the future.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21371093) and the Natural Science Foundation of Jiangsu Province (No. BK20130054).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.04.004.
| [1] | S. Lamansky, P. Djurovich, D. Murphy, et al. Highly phosphorescent bis-cyclometalated iridium complexes:synthesis, photophysical characterization, and use in organic light emitting diodes. J. Am. Chem. Soc. 123 (2001) 4304–4312. DOI:10.1021/ja003693s |
| [2] | T. Sajoto, P.I. Djurovich, A. Tamayo, et al. Blue and near-UV phosphorescence from iridium complexes with cyclometalated pyrazolyl or N-heterocyclic carbene ligands. Inorg. Chem. 44 (2005) 7992–8003. DOI:10.1021/ic051296i |
| [3] | C.H. Yang, M. Mauro, F. Polo, et al. Deep-blue-emitting heteroleptic iridium(Ⅲ) complexes suited for highly efficient phosphorescent OLEDs. Chem. Mater. 24 (2012) 3684–3695. DOI:10.1021/cm010453 |
| [4] | S.B. Meier, W. Sarfert, J .M. Junquera-Hernández, et al., A deep-blue emitting charged bis-cyclometallated iridium(Ⅲ) complex for light-emitting electrochemical cells. J. Mater. Chem. C 1 (2013) 58–68. DOI:10.1039/C2TC00251E |
| [5] | S. Evariste, M. Sandroni, T.W. Rees, et al. Fluorine-free blue-green emitters for light-emitting electrochemical cells. J. Mater. Chem. C 2 (2014) 5793–5804. DOI:10.1039/c4tc00542b |
| [6] | Q. Zhao, M.X. Yu, L.X. Shi, et al. Cationic iridium(Ⅲ) complexes with tunable emission color as phosphorescent dyes for live cell imaging. Organometallics 29 (2010) 1085–1091. DOI:10.1021/om900691r |
| [7] | G.L. Zhang, H.Y. Zhang, Y. Gao, et al. Near-infrared-emitting iridium(Ⅲ) complexes as phosphorescent dyes for live cell imaging. Organometallics 33 (2014) 61–68. DOI:10.1021/om400676h |
| [8] | C. Shi, H.B. Sun, X. Tang, et al. Variable photophysical properties of phosphorescent iridium(Ⅲ) complexes triggered by closo- and nido-carborane substitution. Angew. Chem. Int. Ed. 52 (2013) 13434–13438. DOI:10.1002/anie.201307333 |
| [9] | K.Y. Zhang, J. Zhang, Y.H. Liu, et al. Core-shell structured phosphorescent nanoparticles for detection of exogenous and endogenous hypochlorite in live cells via ratiometric imaging and photoluminescence lifetime imaging microscopy. Chem. Sci. 6 (2015) 301–307. DOI:10.1039/C4SC02600D |
| [10] | S.J. Liu, H. Liang, K.Y. Zhang, et al. A multifunctional phosphorescent iridium(Ⅲ) complex for specific nucleus staining and hypoxia monitoring. Chem. Commun 51 (20105) 7943–7946. |
| [11] | S.J. Liu, J. Zhang, D.F. Shen, et al. Reaction-based phosphorescent nanosensor for ratiometric and time-resolved luminescence imaging of fluoride in live cells. Chem. Commun. 51 (2015) 12839–12842. DOI:10.1039/C5CC04276C |
| [12] | W. Lv, T.S. Yang, Q. Yu, et al. A phosphorescent iridium(Ⅲ) complex-modified nanoprobe for hypoxia bioimaging via time-resolved luminescence microscopy. Adv. Sci 2 (2015) . |
| [13] | L. Murphy, A. Congreve, L.O. Pålsson, J.A.G. Williams. The time domain in costained cell imaging:time-resolved emission imaging microscopy using a protonatable luminescent iridium complex. Chem. Commun. 46 (2009) 8743–8745. |
| [14] | Y.N. Hong, J.W.Y. Lam, B.Z. Tang. Aggregation-induced emission. Chem. Soc. Rev. 40 (2011) 5361–5388. DOI:10.1039/c1cs15113d |
| [15] | M.X. Yu, Q. Zhao, L.X. Shi, et al. Cationic iridium(Ⅲ) complexes for phosphorescence staining in the cytoplasm of living cells. Chem. Commun (2008) 2115–2117. |
| [16] | P. Steunenberg, A. Ruggi, N.S. van den Berg, et al. Phosphorescence imaging of living cells with amino acid-functionalized tris(2-phenylpyridine)iridium(Ⅲ) complexes. Inorg. Chem. 51 (2012) 2105–2114. DOI:10.1021/ic201860s |
| [17] | C. Adachi, R.C. Kwong, P. Djurovich, et al. Endothermic energy transfer:a mechanism for generating very efficient high-energy phosphorescent emission in organic materials. Appl. Phys. Lett. 79 (2001) 2082–2084. DOI:10.1063/1.1400076 |
| [18] | T.Y. Li, X. Liang, L. Zhou, et al. N-heterocyclic carbenes:versatile second cyclometalated ligands for neutral iridium(Ⅲ) heteroleptic complexes, Inorg. Chem 54 (2015) 161–173. |
| [19] | A. Tsuboyama, H. Iwawaki, M. Furugori, et al. Homoleptic cyclometalated iridium complexes with highly efficient red phosphorescence and application to organic light-emitting diode. J. Am. Chem. Soc. 125 (2003) 12971–12979. DOI:10.1021/ja034732d |
| [20] | M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al. Gaussian 09, Revision A.01, Gaussian, Inc. Wallingford, CT (2009) . |
| [21] | S. Lamansky, P. Djurovich, D. Murphy, et al. Synthesis and characterization of phosphorescent cyclometalated iridium complexes. Inorg. Chem. 40 (2001) 1704–1711. DOI:10.1021/ic0008969 |
| [22] | Y.C. Zhu, L. Zhou, H.Y. Li, et al. Highly efficient green and blue-green phosphorescent OLEDs based on iridium complexes with the tetraphenylimidodiphosphinate ligand. Adv. Mater. 23 (2011) 4041–4046. DOI:10.1002/adma.v23.35 |
| [23] | Q.L. Xu, C.C. Wang, T.Y. Li, et al. Syntheses, photoluminescence, and electroluminescence of a series of iridium complexes with trifluoromethyl-substituted 2-phenylpyridine as the main ligands and tetraphenylimidodiphosphinate as the ancillary ligand. Inorg. Chem. 52 (2013) 4916–4925. DOI:10.1021/ic302510p |
| [24] | K.P.S. Zanoni, B.K. Kariyazaki, A. Ito, et al. Blue-green iridium(Ⅲ) emitter and comprehensive photophysical elucidation of heteroleptic cyclometalated iridium(Ⅲ) complexes. Inorg. Chem. 53 (2014) 4089–4099. DOI:10.1021/ic500070s |
 2016, Vol. 27
2016, Vol. 27 


