b School of Physics and Optoelectronic Engineering, Guangdong University of Technology, Guangzhou 510006, China ;
c Key Laboratory of Ecophysics and Department of Physics, School of Science, Shihezi University, Shihezi 832003, China
With more serious environmental pollution, semiconductor nanostructures have attracted intensive interest because of their enormous potential in catalytic applications [1-4]. Zinc tellurium (ZnTe) as an important Ⅱ-Ⅳ semiconductor with a suitable direct band gap of 2.26 eV has been extensively researched in photocatalysis [5-8]. However, due to the fast electron/hole recombination rate, nanostructured ZnTe exhibits poor photocatalytic performance, especially under visible-light irradiation. Many attempts were used to promote electrons transfer to decrease electron/hole recombination. Among them, the fabrication of ZnTe based composites, such as ZnTe/TiO2 nanotube arrays, was considered an effective remedial method [9]. However, the synthesis process is tedious and an unsatisfactory photocatalytic performance under visible-light irradiation is still urgently needed to be solved. Hence, it is essential to find an easier method and more suitable material to fabricate ZnTe based composites with better photocatalytic performance under visible-light irradiation.
Reduced graphene oxide (RGO) has attracted extensive attention because of its peculiar properties, such as low density, high surface area, large open pores [10, 11]. So far, a great number of inorganic nanostructures, such as Cu, MoO3, TiO2, ZnO, ZnSe, CdS, and MoS2, have been compounded with reduced graphene oxide to enhance photocatalysis and achieve better photocatalytic performance since RGO, as building blocks, can promote the separation of the photo-generated electrons and holes [12-15]. However, these materials composited with RGO usually need two steps [16-18].
Herein, we report a one-pot, solvothermal synthetic method for ZnTe/RGO nanocomposites with excellent photodegradation efficiency under visible-light irradiation. In this method, the composite material is obtained by using sodium tellurite (NaTeO3), zinc acetate dihydrate [Zn(CH2COOH)2·2H2O], graphene oxide (GO) sheets and hydrazine hydrate (N2H4·H2O) as precursors, Along with the formation of ZnTe nanoparticles, hydrazine hydrate also reduces GO to RGO. The nanocomposite integrates the advantages of each component material, as it exhibits not only high visible-light photodegradation efficiency, but also excellent visible-light absorption.
2. ExperimentalAll reagents were used without any further purification. GO (18 mg) was dispersed into deionized water (8 mL), and then subjected to ultrasound agitation for at least 2 h. Separately, Zn(CH3COO)2·2H2O (0.15 mmol) was also added to deionized water (5 mL). Then, the second solution was added dropwise to the first and the mixed solution was continued for 2 h in a magnetically stirred water bath (60 ℃) to uniformly deposit Zn2+ on the surface of graphene oxide. Then, Na2TeO3 (0.15 mmol) dispersed in 5 mL deionized water, hydrazine hydrate (5 mL) and ethanolamine (15 mL) were added sequentially to the mixed solution. After stirring for 0.5 h, the suspension was transferred into a 50 mL Teflon-lined autoclave, and the autoclave was sealed and maintained at 200 ℃ for 24 h. After cooling to r.t., the formed black floccules were filtered and washed with deionized water and absolute ethanol several times. Finally, the sample was obtained by drying for 6 h at 60 ℃ under vacuum. Pure ZnTe nanoparticle was prepared following the same procedure, but GO was not added. Similarly, Na2TeO3 and Zn(CH3COO)2·2H2O were not added when prepared pure RGO.
The X-ray diffraction patterns of samples were observed by a Bruker D8 Advance Diffractometer with Cu Ka radiation (λ=0.15418 nm) and the diffraction data were recorded for 2θ angles between 10° and 80°. The morphology of samples was characterized by scanning electron microscope (SEM, LEO1430VP) and transmission electron microscope (TEM, Tecnai G2 F20). Infrared spectra of the samples were determined on a FTIR spectrometer (Bruker VERTEX70). Raman spectra were collected on a Raman spectrometer (Bruker, SENTERRA). Surface electronic states of the samples were measured by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI) with monochromatized Al Ka radiation using C 1s (284.8 eV) as the reference. The optical properties of samples were obtained by using photoluminescence spectra measured on a fluorescence spectrophotometer (Hitachi F-4500) using an excitation wavelength of 250 nm, scanning rate of 1200 nm/min and photomultiplier tube voltage of 700 V. The UV-vis spectra were recorded on an Hitachi U-3010 Spectrophotometer.
In each experiment, 50 mg of photocatalyst was added to 50 mL of MB solution with a concentration of 25 mg/L. After adsorption/ desorption equilibrium in the dark for 120 min, the solution was illuminated by a 300 W UV lamp or an 800 W Xe lamp with a cutoff filter (k≥420 nm) to remove UV light. Circulating water was used to cool the solution to prevent solvent evaporation. During the light irradiation, about 4 mL of the suspension was collected every 20 or 40 min and after centrifugation, the concentration of MB solution was analyzed by measuring the intensity at 664 nm with a UV-vis spectrophotometer.
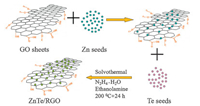
3. Results and discussionThe proposed scheme of the ZnTe/RGO nanocomposites is displayed in Fig. 1. First, Zn2+ is absorbed on the GO sheets by the electrostatic forces. Second, along with Te4+ is reduced to Te2-, hydrazine hydrate reduces GO to RGO. Finally, ZnTe/RGO nanocomposites are successfully synthesized by solvothermal treatment.
|
Download:
|
| Figure 1. A proposed scheme of the formation of ZnTe/RGO nanocomposites. | |
Fig. 2 shows the XRD patterns of the ZnTe and ZnTe/RGO samples. The diffraction peaks at 25.2°, 29.3°, 41.8°, 49.5°, 51.9°, 60.6°, 66.7°, 68.8° and 76.4° are assigned to the (1 1 1), (2 0 0), (2 2 0), (3 1 1), (2 2 2), (4 0 0), (3 3 1), (4 2 0) and (4 2 2) planes of cubic sphalerite ZnTe (JCPDS, Card No. 65-0385). However, no RGO diffraction peaks are found in the ZnTe/RGO samples. The structural characteristics of the ZnTe/RGO nanocomposites and pure GO sheets are further investigated by Raman spectroscopy, as shown in Fig. 2(b). The Raman spectra of ZnTe/RGO nanocomposites reveals two bands at 1337 cm-1 and 1571 cm-1 corresponding to the diamondoid (D) and graphite (G) bands, respectively (the D band comes from defects such as disordered carbon and the G band corresponds to sp-2 bonded carbon [19, 20]). The intensity ratio (ID/IG) of D and G band is 0.9 in GO and lower than 1.2 in ZnTe/ RGO, which is ascribed to the removal of oxygen functionalities and a partially ordered crystal structure of graphene oxide sheets [21]. Hence, we conclude that the cubic sphalerite ZnTe nanoparticles have been successfully deposited on the reduced graphene oxide sheets through the solvothermal treatment.

|
Download:
|
| Figure 2. (a) XRD patterns of ZnTe and ZnTe/RGO samples, (b) Raman spectra of GO and ZnTe/RGO samples. | |
The chemical states of the ZnTe/RGO samples are characterized by XPS spectra to further confirm the formation of RGO. As shown in Fig. 3(a), the C1s XPS spectra for the GO and ZnTe/RGO samples shows four peaks (Table 1) corresponding to the C=C, C-OH, C-O-C and HO-C=O band [22, 23]. Compared with GO [Fig. 3(c)], the area percentages relative to C-C bonds of C-O-C band of ZnTe/RGO (Table 1) are obviously decreased. In Fig. 3(b), two peaks at 532.6 eV and 530.9 eV in the O 1s XPS spectra for ZnTe/RGO sample are attributed to C=O and C-OH bands, respectively [24]. In comparison, only one peak at 532.6 eV is found in pure GO [Fig. 3(d)]. The diminution of corresponding area percentages (Table 1) and appearance of new peak in ZnTe/RGO samples can be assigned to some oxygen-containing functional groups of GO removed after reduction by hydrazine hydrate [25]. The result is consistent with the result of the Raman spectra. Therefore, these results also confirm the successful formation of the RGO sample.

|
Download:
|
| Figure 3. (a) XPS spectra of the C 1s region for ZnTe/RGO, (b) XPS spectra of the O 1s region for ZnTe/RGO, (c) XPS spectra of the C 1s region for GO, (d) XPS spectra of the O 1s region for GO. | |
|
|
Table 1 XPS data (C 1s) of GO and ZnTe/RGO samples of the four main peaks, binding energies and % area normalized to C-C bond in parentheses. |
The morphology of the samples is analyzed by the typical scanning electron microscope (SEM) and transmission electron microscopy (TEM). As shown in Fig. 4(a), pure ZnTe without added GO are stacked randomly. In contrast, a great number of ZnTe nanoparticles are distributed uniformly on the wrinkled sheets in ZnTe/RGO composites [Fig. 4(b)]. Obviously, it suggests that the RGO sheets may be beneficial to prevent the aggregation of ZnTe nanoparticles [24]. The HRTEM image [Fig. 4(c)] and the corresponding fast Fourier transform (FFT) image show 0.35 nm fringe spacing matching with the (1 1 1) crystallographic plane of cubic sphalerite ZnTe, which is consistent with the XRD results of ZnTe.

|
Download:
|
| Figure 4. (a) SEM image of ZnTe samples, (b) TEM image of ZnTe/RGO samples, (c) HRTEM image of ZnTe/RGO samples. | |
Fig. 5(a) shows the Fourier transform infrared spectroscopy (FTIR) of ZnTe/RGO and ZnTe. The absorption bands at 1633 cm-1 and 3437 cm-1 can be attributed to bending vibrations and stretching of hydroxyl groups, respectively [26]. The peak at around 1150 cm-1 can be attributed to the C-O stretching vibration [27]. The peak at 1550 cm-1 is assigned to the graphitic structure of RGO sheets [28]. Thereby, the FTIR results further demonstrate that GO has been reduced.

|
Download:
|
| Figure 5. (a) FTIR spectra of samples, (b) UV-vis spectra of samples. | |
The UV-vis absorption spectra of ZnTe nanoparticles and ZnTe/ RGO nanocomposites are investigated in Fig. 5(b). Compared to ZnTe or RGO samples, light absorbance of ZnTe/RGO nanocomposites is stronger than pure phase ZnTe or RGO at the photoresponse range. It implies that the introduction of RGO into the ZnTe/RGO nanocomposites is able to effectively promote visible light absorption of the nanocomposites [29].
Fig. 6 displays the results of the degradation of methyl blue (MB) solution in a series of experimental conditions. Fig. 6(a) shows the degradation/removal of MB at 120 min by UV irradiation. The bleaching of MB by the ZnTe/RGO nanocomposites reaches 51%, while ZnTe is only about 19%. Fig. 6(b) shows the photocatalytic performance of ZnTe/RGO nanocomposites under visible-light irradiation. Obviously, pure ZnTe shows no photocatalytic activity. In order to ensure the enhanced photocatalysis coming from synergistic effect of ZnTe and RGO, 11.4 mg RGO (calculate from weight ration of ZnTe/RGO) is added into 50 mL of MB solution to test photocatalytic performance. It is clear that pure RGO has better absorption ability but desorption occurs under visible-light irradiation. Hence, ZnTe nanoparticles dispersed uniformly on RGO can effectively enhance photocatalytic perfor mance under visible-light irradiation. Fig. 6(c) shows that the degradation/removal of MB by ZnTe/RGO reaches to 56% after 240 min visible-light irradiation. As shown in Fig. 6(d), ZnTe/RGO displays a similar activity after three cycles of reuse under visible light irradiation, indicatingthestabilityofthenanoheterostructure photocatalysts.

|
Download:
|
| Figure 6. (a) Comparison of MB degradation over different samples under UV light absorbance at a particular time. The initial concentration of MB after adsorption equilibrium are denoted as C and C0, respectively. (b) MB Photodegradation over RGO, ZnTe and ZnTe/RGO samples, (c) comparison of MB degradation over different samples under visible-light irradiation, (d) photocatalytic activity of the ZnTe/RGO for MB degradation with three cycles of usage under visible-light irradiation. | |
Photoluminescence (PL) measurement is used for detecting the separation and recombination of photo-generated charge carriers, and the transfer of photo-generated electrons and holes. Fig. 7(a) shows the PL spectra of ZnTe and ZnTe/RGO samples under 250 nm excitation wavelength at r.t. It is clear that the PL intensity of ZnTe/ RGO is much lower than that in ZnTe, which indicates an increase in the electron/hole separation rate. Hence, the introduction of RGO is advantageous to increase the charge separation efficiency and improve photocatalytic activity [30].

|
Download:
|
| Figure 7. (a) PL spectra of ZnTe/RGO and ZnTe samples (λex=250 nm), (b) schematic diagrams of the photoinduced charge separation and migration processes. | |
The mechanism of enhanced photocatalytic performance of ZnTe/RGO samples is shown in Fig. 7(b). The conduction band (CB) minimum of ZnTe (ca. -1.81 V vs. SHE) is much more negative than the RGO (ca. -0.08 V vs. SHE) [31, 32]. Hence, electrons in the CB of ZnTe can be transferredto RGO easily, whichdecrease the electron/ hole recombination rate of ZnTe. The electrons accumulated on the surface of RGO converts the dissolved oxygen molecules in aqueous solution to highly oxidative species, which can cause the oxidative decomposition of MB effectively [33].
4. ConclusionIn conclusion, ZnTe/RGO nanocomposites were synthesized successfully by using a one-pot, facile, solvothermal approach. TEM results explicitly revealed that cubic sphalerite ZnTe nanoparticles were distributed on the wrinkled RGO sheets. The introduction of the RGO decreased the recombination rates of photo-generated electron/hole pairs and strongly enhanced visible-light absorption resulting in the improvement of photodegradation efficiency. The high photocatalytic activity of the ZnTe/RGO nanocomposites may have a potential photocatalytic application in wastewater treatment.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 11164026, 51172193, 11504313, 51362026) and the Natural Science Foundation for Distinguished Young Scholars of Xinjiang (No. 2013711007).
| [1] | Kumar S., Surendar T., Baruah A., Shanker V.. Synthesis of a novel and stable gC3N4-Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. A 1 (2013) 5333–5340. DOI:10.1039/c3ta00186e |
| [2] | Wang S.P., Li W., Dong Y.Y., Zhao Y.J., Ma X.B.. Effects of potassium promoter onthe performance of PdCl2-CuCl2/AC catalysts for the synthesis of dimethyl carbonate from CO and methyl nitrite. Chin. Chem. Lett. 26 (2015) 1359–1363. DOI:10.1016/j.cclet.2015.06.008 |
| [3] | Zhang L.H., Yang H.Q., Yu J., et al. Controlled synthesis and photocatalytic activity of ZnSe nanostructured assemblies with different morphologies and crystalline phases. J. Phys. Chem. C 113 (2009) 5434–5443. DOI:10.1021/jp810385v |
| [4] | Wu Z.C., Wang H., Xue Y.J., Li B.E., Geng B.Y.. ZnO nanorods/ZnSe heteronanostructure arrays with a tunable microstructure of ZnSe shell for visible light photocatalysis. J. Mater. Chem. A 2 (2014) 17502–17510. DOI:10.1039/C4TA02989E |
| [5] | Wu X.P., Gu J., Zhou S.M., et al. Red bayberry-like ZnTe microstructures:controlled synthesis, growth mechanism and enhanced photocatalytic performance. J. Alloys Compd. 627 (2015) 166–173. DOI:10.1016/j.jallcom.2014.11.199 |
| [6] | Ehsan M.F., He T.. In situ synthesis of ZnO/ZnTe common cation heterostructure and its visible-light photocatalytic reduction of CO2 into CH4. Appl. Catal., B:Environ. 166-167 (2015) 345–352. DOI:10.1016/j.apcatb.2014.11.058 |
| [7] | Sun Y.H., Zhao Q., Gao J.Y., et al. In situ growth, structure characterization, and enhanced photocatalysis of high-quality, single-crystalline ZnTe/ZnO branched nanoheterostructures. Nanoscale 3 (2011) 4418–4426. DOI:10.1039/c1nr10922g |
| [8] | Ehsan M.F., Ashiq M.N., He T.. Hollow and mesoporous ZnTe microspheres:synthesis and visible-light photocatalytic reduction of carbon dioxide into methane. RSC Adv. 5 (2015) 6186–6194. DOI:10.1039/C4RA13593H |
| [9] | Liu Y.T., Zhang X.L., Liu R.H., et al. Fabrication and photocatalytic activity of highefficiency visible-light-responsive photocatalyst ZnTe/TiO2 nanotube arrays. J. Solid State Chem. 184 (2011) 684–689. DOI:10.1016/j.jssc.2011.01.024 |
| [10] | Liu W.J., Cai J.Y., Li Z.H.. Self-Assembly of semiconductor nanoparticles/reduced graphene oxide (RGO) composite aerogels for enhanced photocatalytic performance and facile recycling in aqueous photocatalysis. ACS Sustainable Chem. Eng. 3 (2015) 277–282. DOI:10.1021/sc5006473 |
| [11] | Jiang H., Dai Y.H., Hu Y.J., Chen W.N., Li C.Z.. Nanostructured ternary nanocomposite of rGO/CNTs/MnO2 for high-rate supercapacitors. ACS Sustainable Chem. Eng. 2 (2014) 70–74. DOI:10.1021/sc400313y |
| [12] | Bai S.L., Chen C., Luo R.X., Chen A.F., Li D.Q.. Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sens. Actuators, B:Chem. 216 (2015) 113–120. DOI:10.1016/j.snb.2015.04.036 |
| [13] | Bera R., Kundu S., Patra A.. 2D hybrid nanostructure of reduced graphene oxide-CdS nanosheet for enhanced photocatalysis. ACS Appl. Mater. Interfaces 7 (2015) 13251–13259. DOI:10.1021/acsami.5b03800 |
| [14] | Jin C.J., Cui X.Q., Tian H.W., et al. Photo-less catalysis of TiO2-reduced graphene oxides. Chem. Phys. Lett. 608 (2014) 229–234. DOI:10.1016/j.cplett.2014.06.007 |
| [15] | Liu H., Lv T., Wu X.H., Zhu X.C., Zhu Z.F.. Preparation and enhanced photocatalytic activity of CdS@RGO core-shell structural microspheres. Appl. Surf. Sci. 305 (2014) 242–246. DOI:10.1016/j.apsusc.2014.03.045 |
| [16] | Li Z.Q., Wang H.L., Zi L.Y., Zhang J.J., Zhang Y.S.. Preparation and photocatalytic performance of magnetic TiO2-Fe3O4/graphene (RGO) composites under VISlight irradiation. Ceram. Int. 41 (2015) 10634–10643. DOI:10.1016/j.ceramint.2015.04.163 |
| [17] | Uddin A.S.M.I., Lee K.W., Chung G.S.. Acetylene gas sensing properties of an Agloaded hierarchical ZnO nanostructure-decorated reduced graphene oxide hybrid. Sens. Actuators, B:Chem. 216 (2015) 33–40. DOI:10.1016/j.snb.2015.04.028 |
| [18] | Rajesh U.C., Wang J.F., Prescott S., Tsuzuki T., Rawat D.S.. RGO/ZnO nanocomposite:an efficient, sustainable, heterogeneous, amphiphilic catalyst for synthesis of 3-substituted indoles in water. ACS Sustainable Chem. Eng. 3 (2015) 9–18. DOI:10.1021/sc500594w |
| [19] | Ullah K., Kim Y.H., Lee B.E., et al. Visible light induced catalytic properties of CdSegraphene nanocomposites and study of its bactericidal effect. Chin. Chem. Lett. 25 (2014) 941–946. DOI:10.1016/j.cclet.2014.03.050 |
| [20] | Xiang Q.J., Yu J.Q., Jaroniec M.. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 3 (2011) 3670–3678. DOI:10.1039/c1nr10610d |
| [21] | Liu L., Kou J.H., Guo D.M., et al. Synthesis of thiol-functionalized TiO2 nanocomposite and photocatalytic degradation for PAH under visible light irradiation. Chin. Chem. Lett. 20 (2009) 1366–1370. DOI:10.1016/j.cclet.2009.06.026 |
| [22] | Huang J., Chang Q., Ding Y.B., Han X.Y., Tang H.Q.. Catalytic oxidative removal of 2, 4-dichlorophenol by simultaneous use of horseradish peroxidase and graphene oxide/Fe3O4 as catalyst. Chem. Eng. J. 254 (2014) 434–442. DOI:10.1016/j.cej.2014.05.136 |
| [23] | Wang P., Jiang T.F., Zhu C.Z., et al. One-step, Solvothermal synthesis of grapheneCdS and graphene-ZnS quantum dot nanocomposites and their interesting photovoltaic properties. Nano Res. 3 (2010) 794–799. DOI:10.1007/s12274-010-0046-0 |
| [24] | Pan S.G., Liu X.H.. ZnS-graphene nanocomposite:synthesis, characterization and optical properties. J. Solid State Chem. 191 (2012) 51–56. DOI:10.1016/j.jssc.2012.02.048 |
| [25] | Ray S.C., Bhunia S.K., Saha A., Jana N.R.. Graphene oxide (GO)/reduced-GO and their composite with conducting polymer nanostructure thin films for nonvolatile memory device. Microelectron. Eng. 146 (2015) 48–52. DOI:10.1016/j.mee.2015.04.001 |
| [26] | Jia Z.F., Chen T.D., Wang J., et al. Synthesis, characterization and tribological properties of Cu/reduced graphene oxide composites. Tribol. Int. 88 (2015) 17–24. DOI:10.1016/j.triboint.2015.02.028 |
| [27] | Song H.J., Li N.. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive. Appl. Phys. A 105 (2011) 827–832. DOI:10.1007/s00339-011-6636-1 |
| [28] | Pham T.A., Kim J.S., Kim J.S., Jeong Y.T.. One-step reduction of graphene oxide with L-glutathione. Colloids Surf., A:Physicochem. Eng. Aspects 384 (2011) 543–548. DOI:10.1016/j.colsurfa.2011.05.019 |
| [29] | Xue L.P., Shen C.F., Zheng M.B., et al. Hydrothermal synthesis of graphene-ZnS quantum dot nanocomposites. Mater. Lett. 65 (2011) 198–200. DOI:10.1016/j.matlet.2010.09.087 |
| [30] | Gupta B., Melvin A.A., Matthews T., et al. Facile gamma radiolytic methodology for TiO2-rGO synthesis:effect on photo-catalytic H2 evolution. Int. J. Hydrogen Energy 40 (2015) 5815–5823. DOI:10.1016/j.ijhydene.2015.02.102 |
| [31] | Hajishafiee H., Sangpour P., S. N.. Tabrizi, Facile synthesis and photocatalytic performance of WO3/rGO nanocomposite for degradation of 1-naphthol. Nano 10 (2015) 1550072. DOI:10.1142/S1793292015500721 |
| [32] | Liu W.J., Cai J.Y., Ding Z.X., Li Z.H.. TiO2/RGO composite aerogels with controllable and continuously tunable surface wettability for varied aqueous photocatalysis. Appl. Catal., B:Environ. 174-175 (2015) 421–426. DOI:10.1016/j.apcatb.2015.03.041 |
| [33] | Wei S.H., Wu R., Jian J.K., et al. Graphene oxide/core-shell structured TiO2@TiO2-x nanocomposites with highly efficient visible-light photocatalytic performance. RSC Adv. 5 (2015) 40348–40351. DOI:10.1039/C5RA01458A |
 2016, Vol. 27
2016, Vol. 27 



