b State Key Laboratory of Fine Chemicals, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China ;
c State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116012, China
Fluorescence imaging has unique advantages to study cellular functional molecules in vitro and/or in vivo [1-3].By attaching a sub-cellular organelle specific group, fluorescent probes can be used to detect the localization and monitor the movement of target biomolecules [4-7].Lysosomes are round-shaped organelles that contain acid hydrolase enzymes that break down waste materials and cellular debris.The interior of the lysosomes is acidic at pH 4.8 compared to the slightly basic cytosol at pH 7.2.Lysosomes were traditionally treated only as the cell recycling centers.They can make the senescent cellular debris and needless macromolecules into small molecules, and then the cells utilize the small molecules for living [8].In recent years, lysosomes have been recognized as an more important organelle in cells.They are related to many diseases such as cancer, neurodegenerative disorders, and cardiovascular diseases.It is extremely urgent to make real-time detection and imagining of lysosomes as well as inside molecules.In recent years, fluorescent probes for staining lysosomes [9-11] or image lysosomal H2S [12], NO, [13] HOCl [14] and pH [15] have been reported.
Nitric oxide (NO) as a uncharged free radical exists everywhere in space [16, 17].Because of its highly chemical reactivity, early studies recognized NO as a kind of poison without other biological significance.As time goes on, we started to realize that NO is an indispensable messenger molecule in the cardiac disorders, immune system disorder and neurodegeneration [18-20].It makes white blood cells kill tumor cells and bacteria, and much like neurotransmitter, it can dilate blood vessels.Disclosing the distribution of NO and its relationship with bodily agents will be critical for the treatment for the disorders mentioned above.Some fluorescent probes, including o-phenylenediamine based fluorescent probes [21-29] and copper complex based compounds [30], have been reported to detect NO with some successful applications to image NO in living cells.
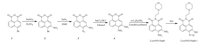
In this paper, we reported a new fluorescent probe LysoNO-Naph to detect NO in lysosomes.Derived from 4-bromo-1, 8-naphthalic anhydride, the NO reactive site o-phenylenediamine and a lysosome target group 4-(2-aminoethyl)-morpholine were introduced to the fluorophore.This probe can be easily obtained with high yield.As shown in Scheme 1, LysoNO-Naph reacted with NO to form a triazole ring and simultaneously displayed a turn-on blue fluorescence.Its propreties of imaging lysosomal NO were also studied.The experimental details are given in Supporting information.
|
Download:
|
| Scheme. 1. Synthesis of LysoNO-Naph and the reaction process with NO. | |
2. Experimental 2.1. Materials and instruments
Unless otherwise noted, all reagents were obtained from Aldrich and used without further purification.1H NMR and 13C NMR spectra were recorded on a Burker 400 MHz spectrometer.UV-visible absorption spectra were collected on Agilent Cary 60 UV/VIS spectrophotometer.Fluorescence emission spectra were performed on Cary Eclipse Fluorescence Spectrophotometer (Serial No.FL0812-M018).Compounds 2-4 were synthesized with a modified method according to [31].
HPLC-MS analysis was performed on Agilent 6540 UHD Accurate-Mass Q-TOF LC/MS using an HPLC system composed of a pump (Agilent ZORBAX Eclipse Plus C18 2.1 mm x 50 mm) and a LysoNO-Naph detector (254 nm).
2.2. Synthesis of compound 2-4 2.2.1. Synthesis of compound 2NaNO3 (2.0 g, 23.53 mmol) was carefully added to a suspension of 4-bromo-1, 8-naphthalic anhydride (5.0 g, 18.1 mmol) in concentrated sulphuric acid (30 mL).The solution was stirred for 3 h at 0 ℃, and then moved to room temperature for 1 h.The solution was poured into 300 mL of ice-water, filtered to give yellow solid.The crude product was recrystallized in glacial acetic acid to yield product as yellow solid (3.76 g, 65%).1H NMR (400 MHz, DMSO-d6): δ 8.93 (s, 1H), 8.84 (d, 1H, J=8.6 Hz), 8.75 (d, 1H, J=7.9 Hz), 8.23-8.16 (m, 1H).MS (EI) calcd.for C12H4BrNO5 [M+] 320.93, found 320.93.
2.2.2. Synthesis of compound 3After a mixture of compound 2 (2 g, 6.32 mmol) and DMF (25 mL) was stirred at room temperature for 10 minutes, NaN3 (0.608 g, 9.35 mmol) was carefully added, and the mixture was stirred for 3 h at room temperature.The solution was poured into 200 mL of ice-water, and the precipitate was filtered and washed with water.After drying, compound 3 was obtained (1.2 g, 68%).1H NMR (400 MHz, DMSO-d6): δ 8.69 (d, 1H, J=8.5 Hz), 8.34 (d, 1H, J=7.2 Hz), 8.08 (s, 1H), 7.69 (t, 1H, J=7.9 Hz).MS (EI) calcd.for C12H4N4O5 [M+] 284.02, found 284.02.
2.2.3. Synthesis of compound 4After a mixture of SnCl2 (3 g, 13.31 mmol) and concentrated hydrochloric acid (8 mL) was stirred at room temperature for 10 min, compound 3 was slowly added, and the mixture was stirred for 40 minutes at 50 ℃.Then stirred at 80 ℃ with ethanol (8 mL) for another 3 h.After the reaction, mixture was cooled down to room temperature, the precipitate was filtered and washed with water.After drying, red compound 4 was obtained (0.54 g, 81‰).1H NMR (400 MHz, DMSO-d6): δ 8.58 (d, 1H, J=8.5 Hz), 8.21 (d, 1H, J=7.1 Hz), 7.89 (s, 1H), 7.62-7.56 (m, 1H), 6.89 (s, 2H), 5.28 (s, 2H). MS (ESI) calcd. for C12H9N2O3 [M+ H+] 238.13, found 238.13.
2.3. Synthesis of LysoNO-NaphTo a solution of 3, 4-diamino-1, 8-naphthalic anhydride (200 mg, 0.88 mmol) in 20 mL 2-methoxyethanol was added 2.0 eq 4-(2-aminoethyl)-morpholine (231 mL, 1.76 mmol).The mixture was then heated at 125 ℃ for 5 h and monitored by TLC.After the completion of the reaction, the residue was purified by silica gel column (CH2Cl2:CH3OH=20:1) to give 150 mg LysoNO-Naph as red powder in 51% yield.M.p.225-227 ℃.1H NMR (400 MHz, DMSO-d6): δ 8.49 (d, 1H, J=8.3 Hz), 8.20 (d, 1H, J=7.0 Hz), 7.92 (s, 1H), 7.58-7.52 (t, 1H), 6.51 (s, 2H), 5.15 (s, 2H), 4.14 (t, 2H, J=6.9 Hz), 3.56-3.51 (m, 4H), 2.53-2.50 (m, 2H), 2.45 (s, 4H).13C NMR (100 MHz, DMSO-d6): δ 164.50 (s), 163.57 (s), 136.88 (s), 131.00 (s), 128.47 (s), 127.70 (s), 124.19 (s), 123.75 (s), 122.11 (s), 121.31 (s), 120.06 (s), 108.76 (s), 66.68 (s), 56.32 (s), 53.89 (s), 36.82 (s).HRMS (ESI) calcd.for C18H21N4O3 [M+ H+] 341.1614, found 341.1610.
2.4. Culture of CHO cells and fluorescent imaging:CHO cells was hatched in an atmosphere of 5% CO2 and 95% air in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) at 37 ℃.The cells were seeded in 24-well flat-bottomed plates and then incubated for 72 h at 37 ℃ under 5% CO2. After adding LysoNO-Naph (5 μmol/L) to the cells, incubate them for another 30 min.Then use phosphate-buffered saline (PBS, 10 mmol/L) to wash cells for three times.Added 100 mmol/L of SNP (release NO, 50 μmol/L) and Neutral Red (2 μmol/L) to the cells, incubate them for 30 min.Fluorescence imaging was observed under a confocal microscopy (Olympus FV1000) with a 60x objective lens.
3. Results and discussionAs lysosomes have an acid interior with pH from 4.0 to 6.0, lysosome-targetable fluorescent probes must remain unaffected fluorescence without the interaction with analyte at least in this pH range.So, the pH effect on the absorption and fluorescence of LysoNO-Naph were firstly studied.As shown in Fig. 1, the maximum absorption peak of LysoNO-Naph at 462 nm remained stable in the pH range from pH 3.80 to 12.54.Accordingly, LysoNO-Naph did not fluoresce in this pH range.In much more acidic solutions, the maximal absorption peak moved to shorter wavelength.And in much more basic solutions, the maximal absorption peak reminded but with decreased fluorescence intensity.Hence, LysoNO-Naph is suitable to be applied to monitor lysosomal NO, since it is stable in pH from 4.0 to 6.0 without any changes.The polarity effects on absorption and fluorescence were also examined.As indicated in Figs.S2 and S3 (see Supporting information), LysoNO-Naph is a typical intramolecular chargetransfer (ICT) type of fluorescent dyes.With the increase of polarity, the absorption and emission wavelengths would be redshifted.

|
Download:
|
| Figure 1. Effects of pH on the absorption of LysoNO-Naph. | |
The absorption and fluorescence spectra of LysoNO-Naph with the addition of NO were obtained in mixture solutions of acetonitrile and PBS buffer s (1:1, v/v, pH 7.4, 20 mmol/L).LysoNO-Naph has a main absorption band at 462 nm and a second absorption band at 368 nm (Fig. 2a).When 25 equiv.of SNP (NO donor) were added to the solution of LysoNO-Naph, a significant decrease in the 462 nm absorption and a blue-shifted absorption band centered at 368 nm, which was attributed to the formation of compound LysoNO-Naph-1 and increased in intensity, were observed with an isoabsorption point at 405 nm.In the same time, the fluorescence emission band centered at 460 nm appeared and increased in intensity (ΦF=0.23) (Fig. 2b).We found that if the concentration of added SNP was 25 equiv., the reaction can be finished within 20 min (insets in Fig. 2).With much more SNP like 50 equiv.(Fig.S1 in Supporting information), the reaction can be finished in a shorter time (10 min).Then we decided to use 25 equiv.of SNP to examine the performance of LysoNO-Naph in following experiments.

|
Download:
|
| Figure 2. (a) Absorption and (b) fluorescence of LysoNO-Naph (10 μmol/L) with SNP (25 eqiv.) from 0 to 30 min. | |
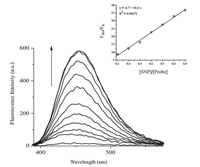
Fig. 3 displayed the titration experiments of LysoNO-Naph with the addition of 0-1 eqiv.SNP.There was a good linearity between the ratio in the fluorescent intensity (F454/F0) and concentrations of SNP (in the range of 0-1 eqiv.) with a detection limit to 4.57 μmol/L (Fig. 3).Moreover, the light blue fluorescence change can be easily recognized by naked eyes.The reaction product was analyzed by HPLC-MS.As shown in Fig. 4, the blue fluorescence product was proved to be LysoNO-Naph-1.
|
Download:
|
| Figure 3. (a) Fluorescence changes and (b) fluorescence ratio (F454/F0) changes of LysoNO-Naph (10 μmol/L) upon addition of SNP (0-1 eqiv.). | |

|
Download:
|
| Figure 4. HPLC-MS of the reaction product of LysoNO-Naph (10 μmol/L) and SNP (25 eqiv.). | |
We also tested the selectivity of LysoNO-Naph with different kind of reactive species (ROS) and reactive nitrogen species (RNS) including NO, NO2-, NO3-, H2O2 and ClO-.As shown in Fig. 5, when LysoNO-Naph was added with 25 equiv.SNP, a great fluorescent increase at 454 nm and a great absorption band at 368 nm were observed.With the addition of 100 equiv.of NO2-, NO3-, H2O2 and ClO-, no obvious changes in fluorescence and absorption were observed.In this regard, LysoNO-Naph has a high selectivity for NO.

|
Download:
|
| Figure 5. (a) Absorption and (b) fluorescence of LysoNO-Naph (10 μmol/L) with the addition of ClO-, H2O2, NO3, NO2-(100 eqiv.) and SNP(25 eqiv.). | |
We next applied LysoNO-Naph for fluorescence imaging of NO in CHO cells (Chinese Hamster Ovary).After incubation with 5 μmol/L LysoNO-Naph in culture medium for 30 min, cells were washed with phosphate buffered saline (PBS, 10 mmol/L, pH 7.4) for three times to remove needless probe.There was a very weak fluorescence existed in confocal image (Fig. 6a).Then 50 μmol/L SNP was added and treated for another 30 min, and a strong blue fluorescence was observed, indicating the detection of cellular NO by LysoNO-Naph (Fig. 6b).In order to identify organelles from the fluorescence imaging of LysoNO-Naph in cells, we then carried out fluorescence localization by co-staining cells with commercially available organelle-specific dyes (Fig. 6c and d).The highly blue fluorescent regions with LysoNO-Naph (Fig. 6b) and signals from NR (Fig. 6c) overlapped perfectly (Fig. 6d), indicating that these fluorescent regions were localized at lysosomes.These experi-ments demonstrated that LysoNO-Naph can be used as a fluorescent probe to detect NO in lysosomes of living cells.

|
Download:
|
| Figure 6. Fluorescence images of CHO cells incubated with 10 μmol/L LysoNO-Naph and NO.Cells treated with LysoNO-Naph (a) in the absence and (b) presence of 50 eqiv.SNP. (c) Image of lysosome tracker (Neutral Red).(d) Fluorescence co-localization imaging of LysoNO-Naph and Neutral Red.(e) Bright field image. | |
4. Conclusion
In conclusion, we have reported a fluorescent probe LysoNO-Naph for imaging NO in lysosomes based on the 1, 8-napthalimide.The probe can be obtained easily with a high yield.Its insensitivity to pH from 3.8-12 made it possible to be used for cellular lysosome imaging.LysoNO-Naph displayed a high selectivity for NO and high sensitivity with a detection limit down to 4.57 μmol/L.
AcknowledgmentWe thank financial supports from the National Natural Science Foundation of China (Nos.21276251, 21506206, 21402191, 21502189), the 100 talents program funded by Chinese Academy of Sciences, Dalian Cultivation Fund for Distinguished Young Scholars (Nos.2014J11JH130, 2015J12JH205) and the National Science Fund for Excellent Young Scholars (No.21422606).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.016.
| [1] | Xu Z.C., Yoon J., Spring D.R.. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 39 (2010) 1996–2006. DOI:10.1039/b916287a |
| [2] | Chan J., Dodani S.C., Chang C.J.. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 4 (2012) 973–984. DOI:10.1038/nchem.1500 |
| [3] | Liu H.Y., Zhao M., Qiao Q.L., et al. Fluorescein-derived fluorescent probe for cellular hydrogen sulfide imaging. Chin. Chem. Lett. 25 (2014) 1060–1064. DOI:10.1016/j.cclet.2014.05.010 |
| [4] | Lee M.H., Park N., Yi C., et al. Mitochondria-immobilized pH-sensitive off-on fluorescent probe. J. Am. Chem. Soc. 136 (2014) 14136–14142. DOI:10.1021/ja506301n |
| [5] | Masanta G., Lim C.S., Kim H.J., et al. A mitochondrial-targeted two-photon probe for zinc ion. J. Am. Chem. Soc. 133 (2011) 5698–5700. DOI:10.1021/ja200444t |
| [6] | Ishida M., Watanabe H., Takigawa K., et al. Synthetic self-localizing ligands that control the spatial location of proteins in living cells. J. Am. Chem. Soc. 135 (2013) 12684–12689. DOI:10.1021/ja4046907 |
| [7] | Dai Y., Lv B.K., Zhang X.F., Xiao Y.. A two-photo mitotracker based on a naphthalimide fluorophore:synthesis, photopysical properties and cell imaging. Chin. Chem. Lett. 25 (2014) 1001–1005. DOI:10.1016/j.cclet.2014.05.020 |
| [8] | Dell' Angelica E.C., Mullins C., Caplan S., S. J.. Bonifacino, Lysosome-related organelles. FASEB J 14 (2000) 1265–1278. DOI:10.1096/fj.14.10.1265 |
| [9] | Zhou K., Wang Y., Huang X., et al. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. Int. Ed. Engl. 50 (2011) 6109–6114. DOI:10.1002/anie.v50.27 |
| [10] | Wang X.H., Nguyen D.M., Yanez C.O., et al. High-fidelity hydrophilic probe for two-photon fluorescence lysosomal imaging. J. Am. Chem. Soc. 132 (2010) 12237–12239. DOI:10.1021/ja1057423 |
| [11] | Li Z., Song Y.L., Yang Y.H., et al. Rhodamine-deoxylactam functionalized poly[styrene-alter-(maleic acid)] s as lysosome activatable probes for intraoperative detection of tumors. Chem. Sci. 3 (2012) 2941–2948. DOI:10.1039/c2sc20733h |
| [12] | Qiao Q.L., Zhao M., Lang H.J., et al. A turn-on fluorescent probe for imaging lysosomal hydrogen sulfide in living cells. RSC Adv. 4 (2014) 25790–25794. DOI:10.1039/c4ra03725a |
| [13] | Yu H.B., Xiao Y., Jin L.J.. A lysosome-targetable and two-photon fluorescent probe for monitoring endogenous and exogenous nitric oxide in living cells. J. Am. Chem. Soc. 134 (2012) 17486–17489. DOI:10.1021/ja308967u |
| [14] | Yuan L., Wang L., Agrawalla B.K., et al. Development of targetable two-photon fluorescent probes to image hypochlorous acid in mitochondria and lysosome in live cell and inflamed mouse model. J. Am. Chem. Soc. 137 (2015) 5930–5938. DOI:10.1021/jacs.5b00042 |
| [15] | Yin L.Y., He C.S., Huang C.S., et al. A dual pH and temperature responsive polymeric fluorescent sensor and its imaging application in living cells. Chem. Commun. 48 (2012) 4486–4488. DOI:10.1039/c2cc30404j |
| [16] | Chameides W.L., Walker J.C.G., Nagy A.F.. Possible chemical impact of planetary lightning in the atmospheres of Venus and Mars. Nature 280 (1979) 820–822. DOI:10.1038/280820a0 |
| [17] | Ziurys L.M., McGonagle D., Minh Y., Irvine W.M.. Nitric oxide in star-forming regions-further evidence for interstellar N-O bonds. Astrophys. J. 373 (1991) 535–542. DOI:10.1086/170072 |
| [18] | Balligand J.L., Kelly R.A., Marsden P.A., Smith T.W., Michel T.. Control of cardiac muscle cell function by an endogenous nitricoxide signaling system. Pro. Natl. Acad. Sci. USA 90 (1993) 347–351. DOI:10.1073/pnas.90.1.347 |
| [19] | Wink D.A., Mitchell J.B.. Chemical biology of nitric oxide:insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 25 (1998) 434–456. DOI:10.1016/S0891-5849(98)00092-6 |
| [20] | Thomas C.E., Darley-Usmar V.. Forum on therapeutic applications of reactive oxygen and nitrogen species in human disease. Free Radic. Biol. Med. 28 (2000) 1449–1450. DOI:10.1016/S0891-5849(00)00254-9 |
| [21] | Gupta N., Reja S.I., Bhalla V., et al. An approach for the selective detection of nitric oxide in biological systems:an in vitra and in vitro perspective. Chem. Asian J. 11 (2016) 1020–1027. DOI:10.1002/asia.v11.7 |
| [22] | Dong X.H., Heo C.H., Chen S.Y., Kim H.M., Liu Z.H.. Quinoline-based two-photon fluorescent probe for nitric oxide in live cells and tissues. Anal. Chem. 86 (2014) 308–311. DOI:10.1021/ac403226h |
| [23] | Zhang H.X., Chen J.B., Guo X.F., Wang H., Zhang H.S.. Highly sensitive lowbackground fluorescent probes for imaging of nitric oxide in cells and tissues. Anal. Chem. 86 (2014) 3115–3123. DOI:10.1021/ac4041718 |
| [24] | Sun Y.Q., Liu J., Zhang H.X., et al. A mitochondria-targetable fluorescent probe for dual-channel NO imaging assisted by intracellular cysteine and glutathione. J. Am. Chem. Soc. 136 (2014) 12520–12523. DOI:10.1021/ja504156a |
| [25] | Wang M., Xu Z.C., Wang X., Cui J.N.. A fluorescent and colorimetric chemosensor for nitric oxide based on 1, 8-naphthalimide. Dyes Pigm. 96 (2013) 333–337. DOI:10.1016/j.dyepig.2012.08.024 |
| [26] | Chen J.B., Zhang H.X., Guo X.F., Wang H., Zhang H.S.. Novel B, O-chelated fluorescent probe for nitric oxide imaging in raw 264.7 macrophages and onion tissues. Anal. Chim. Acta 800 (2013) 77–86. DOI:10.1016/j.aca.2013.09.019 |
| [27] | Zhang H.X., Chen J.B., Guo X.F., Wang H., Zhang H.S.. Highly sensitive determination of nitric oxide in biologic samples by a near-infrared BODIPY-based fluorescent probe coupled with high-performance liquid chromatography. Talanta 116 (2013) 335–342. DOI:10.1016/j.talanta.2013.05.043 |
| [28] | Vegesna G.K., Sripathi S.R., Zhang J.T., et al. Highly water-soluble BODIPY-based fluorescent probe for sensitive and selective detection of nitric oxide in living cells. ACS Appl. Mater. Interfaces 5 (2013) 4107–4112. |
| [29] | Gabe Y., Urano Y., Kikuchi K., Kojima H., Nagano T.. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore-rational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 126 (2004) 3357–3367. DOI:10.1021/ja037944j |
| [30] | Kumar P., Kalita A., Mondal B.. Copper(Ⅱ) complexes as turn on fluorescent sensors for nitric oxide. Dalton Trans. 41 (2012) 10543–10548. DOI:10.1039/c2dt31068f |
| [31] | Li F., Cui J.N., Guo L.Y., et al. Molecular design, chemical synthesis, and biological evaluation of '4-1' pentacyclic aryl/heteroaryl-imidazonaphthalimides. Bioorg. Med. Chem. 15 (2007) 5114–5121. DOI:10.1016/j.bmc.2007.05.032 |
 2016, Vol. 27
2016, Vol. 27 


