b State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China ;
c Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Nankai University, Tianjin 300071, China ;
d The Ural Federal University Named after the First President of Russia B. N. Yeltsin, Ekaterinburg 620002, Russia
Pesticides play an important role in agricultural development in our country. However, pesticide resistance has become a serious concern due to continuous application of a single pesticide with a unique mode of action [1-5], Natural products, often having new modes of action, low-residue and high selectivity, are important pesticide leads [6], and following structure optimization, natural products are strong candidates for the pesticide market [7, 8]. Identifying new lead compounds is imperative for pesticide development [9, 10]. In recent years, the combination of computer simulation, chemical synthesis and biological testing has become the new policy for drug development in the post genomic era [11]. This has an important impact on novel pesticide development.
Transketolase is widely distributed among plants, animals, fungi and bacteria [12], and plays an important role in the calvin cycle of plant photosynthesis [13, 14]. It has potential as a new herbicide target [15]. In a previous study, using computer-aided drug screening technology, an amide compound 12007063, with good biological activity, which targeted transketolase was discovered. Amide compounds are widely used as pesticides. For example, propanil belongs to the photosystem PSII herbicides, and fluopyram which belongs to SDHI (succinate dehydrogenase inhibitors) fungicides is also an amide compound. In this study, a series of N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amide derivatives were designed and synthesized for biological screening from lead compound 12007063 by introducing bioactive substructures into the target molecules using the principle of pesticide molecular designation (Fig. 1).

|
Download:
|
| Figure 1. The structure of herbicide lead compound 12007063. | |
2. Experimental 2.1. Chemistry
Reagents and solvents were analytical grade and the anhydrous reagents were dried by standard methods. The melting points were measured on an XT-4A apparatus and uncorrected. NMR spectra were obtained on a Bruker AV-400 spectrometer operating at 400 MHz for 1H NMR and 100 MHz for 13C NMR. NMR resonances were registered using CDCl3 as solvents and TMS as the internal standard. The high resolution mass spectra were recorded on an Agilent 6520-QTOF LC/MS having ESI source in positive mode.
Synthesis of the title compounds was conducted as shown in Scheme 1. Methyl furan-2-carboxylate (compound 1) was prepared according to literature [16]. Intermediate 2 was prepared via the reaction of methyl furan-2-carboxylate with CH3CN and NaH in toluene by refluxing for 24 h [17]. The cyclization of compound 2 with phenylhydrazine gave intermediate 3. Compounds 5a-5t were obtained by the reaction of 4a-4t with SOCl2 and a catalytic amount of DMF by refluxing. Compounds 6a-6t were synthesized by way of a condensation reaction using Et3N as the base in CH2Cl2. The structures of all newly synthesized compounds were characterized by melting points, 1H NMR, 13C NMR and HRMS.

|
Download:
|
| Scheme. 1. Synthesis of the target compounds. | |
2.2. Biological assay
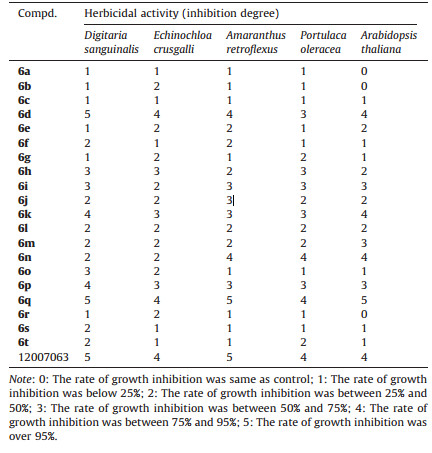
The herbicide activity of the target compounds was evaluated through foliar treatment [18]. The symptoms were monitored at 48 h post-treatment and compared with the control group. The grading standard [19] for the inhibition effect is shown in Table 1. The target plants tested for herbicide efficacy were Digitaria sanguinalis L., Echinochloa crusgalli L., Amaranthus retroflexus L., Portulaca oleracea L. and Arabidopsis thaliana.
|
|
Table 1 Herbicidal activity of test compounds at 1000 μg/mL. |
The fungicide activity of the target compounds was tested using the fungi growth inhibition method [20]. Representative fungi used in this study included Alternaria solani (AS), Botrytis cinerea (BC), Cercospora arachidicola(CA), Gibberella zeae (GZ), Phytophthora infestans (Mont) de Bary (PI), Physalospora piricola (PP), Pellicularia sasakii (PS), Sclerotinia sclerotiorum (SS), and Rhizoctonia cerealis (RC).
3. Results and discussion 3.1. SynthesisHeterocyclic compounds are important lead sources for drug and pesticide development and the methods for their construction are well reported [21-23]. Our former research discovered one compound, containing furan and pyrazole ring, which could be studied as an herbicide lead. This study focused on optimization of this lead and a series of novel N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amides derivatives from 3-(furan-2-yl)-1 -phenyl-H-pyrazol-5-amine (3) was synthesized. We first tried to prepare 3-(furan-2-yl)-1-phenyl-H-pyrazol-5-amine (3) using EtOH as the solvent, and the cycling reaction of 2-furoylacetonitrile with phenylhydrazine was progressed at the reflux temperature. Byproduct was formed and the yield of the desired product was low. However, after optimization of the reaction conditions, the yield was increased from 54.01% to 90.09% [24]. 2-Furoylacetonitrile was allowed to react with phenylhydrazine at 130 ℃ for 1 h, and the desired 3-(furan-2-yl)-1-phenyl-H-pyrazol-5-amine (3) was successfully generated with over 90% yield.
During the synthesis of the target compounds (6), intermediate 3 did not react completely, and the retention factor values of the target compounds and intermediate 3 were very close. It was difficult to separate and purify the target compounds using column chromatography. After washing with HCl, the alkalic intermediate was easily moved, and the purification efficiency of the target compounds by chromatography was significantly improved. The chemical structures of the target compounds were confirmed by spectroscopic data, and these data are given in Supporting information. Here, the spectroscopic data of 6t were given as a representative. 6t: 1H NMR (400 MHz, CDCl3): δ 8.82 (s, 1H, NH), 7.49 (d, 3H, J=7.2 Hz, Ph-H), 7.43 (dd, 3H, J=15.7, 7.6 Hz, Ar-H), 7.09 (s, 1H, Pyrazole-H), 6.90 (d, 1H, J=7.3 Hz, Ph-H), 6.79 (d, 1H, J=8.1 Hz, Ph-H), 6.77 (d, 1H, J=3.5 Hz, Furan -H), 6.67 (d, 1H, J=8.0 Hz, Ph-H), 6.51 (d, 1H, J=1.7 Hz, Furan-H), 4.73 (s, 2H, -OCH2-), 3.04 (s, 2H, CH2), 1.43 (s, 6H, 2× CH3). 13C NMR (101 MHz, CDCl3): δ 165.77, 148.34, 147.67, 144.48, 142.22, 141.68, 137.51, 135.56, 129.80, 129.47, 128.38, 124.57, 120.73, 119.90, 114.59, 111.31, 106.75, 95.44, 88.16, 69.20, 43.07, 28.18. HRMS(ESI) [M+H]+ calcd. for C25H23N3O4: 430.1767, found: 430.1760.
3.2. Biological evaluations 3.2.1. Herbicidal activity of the target compounds.Herbicidal activity of the target compounds was evaluated using the foliar treatment method. The results indicated that (Table 1) all of the target compounds showed moderate to good herbicidal activity against the target weeds at 1000 mg/L. Compound 6d exhibited good herbicidal activity, and the degree of inhibition against D. sanguinalis was 5 which was equal to that of lead compound 12007063. Compound 6q also showed good herbicidal activity against the target weeds, and the inhibition degree against D. sanguinalis, A. retroflexus and A. thaliana was 5, while, for E. crusgalli and P. oleracea, the results were 4 degree. The herbicidal activity of compound 6q against A. thaliana was slightly better than that of lead compound 12007063. Compound 6n showed better activity against broadleaf weeds.
The herbicidal efficacy of the target compounds showed that introducing F, Cl or CF3 into the phenyl ring could improve the herbicidal activity. Further studies found that the herbicidal activity and the weed controlling spectrum of compounds 6d and 6k could be significantly improved by the introduction of CF3 or F to the ortho-positions of the benzene ring. By comparison, introducing CF3 (6p) or a nicotinic acid group (6q) to the pyridine ring of compounds 6p-6s could increase their herbicidal activity and expand their herbicide spectrum. Compounds 6d and 6q had good herbicidal activity and a broad spectrum, and they deserve further investigation. Compound 6n showed better activity against broadleaf targets with good selectivity.
3.2.2. Fungicide activity of the target compoundsAs summarized in Table 2, all of the target compounds showed fungicide activity against the selected fungi in vitro at 50 mg/L. The fungicide activity and the antifungal spectrum of the target compounds were improved when compared with the compound 12007063. All compounds demonstrated moderate to good antifungal activity against S. sclerotiorum with growth inhibition over 60%. Notably, compounds 6f, 6o and 6q demonstrated 100% growth inhibition of S. sclerotiorum. Against P. sasakii, compound 6c presented the highest activity with an inhibition rate of 91%. Compounds 6b and 6t showed the best activity against the strain of C. arachidicola with the inhibition rate of 54%. Compounds 6a and 6g were the most active compounds toward the strain of P. piricola attested by an inhibition rate over 80%. Compound 6a, 6b, 6c, 6g, 6m and 6t presented the good activity against more than one fungi with the inhibition rate was higher than 70%. So the EC50 of these compounds against the corresponding fungi was tested and the results were showed in Table 3.
|
|
Table 2 Fungicidal activity of test compounds (50 μg/mL). |
|
|
Table 3 The EC50 of the selected compounds. |
Comparison of the fungicidal activity of the target compounds illustrated that the introduction of F, Cl or CF3 into the benzene ring could increase antifungal activity and expand the antifungal spectrum. Further studies found that the antifungal activity and spectrum were indeed improved by introducing F (6f) or CF3 (6c) groups into the meta-position at the benzene ring. The antifungal activity of compounds 6l and 6m showed that introduction of Cl did not improve their fungicidal activity. The antifungal activity of compound 6o against the nine fungi was higher than that of compound 6n, which indicated that introducing a furan ring could improve antifungal activity. The antifungal activity of compounds 6p-6s showed that the addition of CF3 to the pyridine ring could expand the antifungal spectrum, but failed to significantly improve its activity. The inhibition rates of compound 6c against S. sclerotiorum and P. sasakii were over 90%, and the EC50 was 17.06 mg/L and 13.94 mg/L, respectively. Hence, 6c deserves further investigation. Compounds 6a and 6g with inhibition rates against P. piricola and S. sclerotiorum reached more than 80%, and the EC50 was lower more than 25 mg/L, could also be used to conduct more indepth studies.
4. ConclusionIn summary, a series of N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amides derivatives were designed and synthesized. The target compounds were characterized by 1H NMR, 13C NMR and HRMS. The bioassays showed that some of the compounds exhibited good herbicidal activity against target weeds. Results of the fungicidal bioassays showed that some compounds presented good antifungal activity with a broad spectrum.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 31171877, 31571991, 21372132) and the International Science & Technology Cooperation Program of China (No. 2014DFR41030).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.06.019.
| [1] | Umina P.A., Weeks A.R., Roberts J., et al. The current status of pesticide resistance in Australian populations of the redlegged earth mite (Halotydeus destructor). Pest Manag. Sci. 68 (2012) 889–896. DOI:10.1002/ps.v68.6 |
| [2] | Norsworthy J.K., Ward S.M., Shaw D.R., et al. Reducing the risks of herbicide resistance:best management practices and recommendations. Weed Sci. 60 (2012) 31–62. DOI:10.1614/WS-D-11-00155.1 |
| [3] | T. Van Leeuwen, W. Dermauw, M. Grbic, L. Tirry, R. Feyereisen, Spider mite control and resistance management:does a genome help? Pest Manag. Sci. 69(2013) 156-159. |
| [4] | H. Sierotzki, G. Scalliet, A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides, Phytopathology 103(2013) 880-887. |
| [5] | Consortium R.E.X.. Heterogeneity of selection and the evolution of resistance. Trends Ecol. Evol. 28 (2013) 110–118. DOI:10.1016/j.tree.2012.09.001 |
| [6] | J.W.H. Li, J.C. Vederas, Drug discovery and natural products:end of an era or an endless frontier? Science 325(2009) 161-165. |
| [7] | Duke S.O., Dayan F.E., Rimando A.M., et al. Invited paper:chemicals from nature for weed management. Weed Sci. 50 (2002) 138–151. DOI:10.1614/0043-1745(2002)050[0138:IPCFNF]2.0.CO;2 |
| [8] | Matsuura B.S., Keylor M.H., Li B., et al. A scalable biomimetic synthesis of resveratrol dimers and systematic evaluation of their antioxidant activities. Angew. Chem. Int. Ed. Engl. 54 (2015) 3754–3757. DOI:10.1002/anie.201409773 |
| [9] | Wang T., Wu M.B., Chen Z.J., et al. Fragment-based drug discovery and molecular docking in drug design. Curr. Pharm. Biotechnol. 16 (2015) 11–25. DOI:10.2174/1389201015666141122204532 |
| [10] | Gengenbacher M., Dick T.. Antibacterial drug discovery:doing it right. Chem. Biol. 22 (2015) 5–6. DOI:10.1016/j.chembiol.2014.12.005 |
| [11] | Lindert S., Li M.X., Sykes B.D., McCammon J.A.. Computer-aided drug discovery approach finds calcium sensitizer of cardiac troponin. Chem. Biol. Drug Des. 85 (2015) 99–106. DOI:10.1111/cbdd.12381 |
| [12] | Zhao B., Huo J.Q., Xing J.H., et al. Homologous modeling of transketolase AtTKL1 and its combination with a-terthienyl in Arabidopsis thaliana. Chem. J. Chin. Univ. 36 (2015) 682–686. |
| [13] | Murphy D.J., Walker D.A.. The properties of transketolase from photosynthetic tissue. Planta 155 (1982) 316–320. DOI:10.1007/BF00429458 |
| [14] | Villafranca J.J., Axelrod B.. Heptulose synthesis from nonphosphorylated aldoses and ketoses by spinach transketolase. J. Biol. Chem. 246 (1971) 3126–3131. |
| [15] | Henkes S., Sonnewald U., Badur R., Flachmann R., Stitt M.. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 13 (2001) 535–551. DOI:10.1105/tpc.13.3.535 |
| [16] | J. Zheng, Y.Q. Liu, J.Y. Lv, Synthesis of methyl-furoate, Fine Chem. Intermed. 37(2007) 41-42, 45. |
| [17] | Ma L.C., Yuan L.W., Xu C.Z., et al. An efficient synthesis of 2-aminothiophenes via the gewald reaction catalyzed by an N-methylpiperazine-functionalized polyacrylonitrile fiber. Synthesis 45 (2013) 45–52. |
| [18] | Zhang J.L., Zhang L.H., Liu Y.C., et al. The herbicidal activity of mutant isolates from Botrytis cinerea. Agric. Sci. China 5 (2006) 622–628. DOI:10.1016/S1671-2927(06)60102-8 |
| [19] | Zhang L.H., Kang Z.H., Xu J., Xu W.C., Zhang J.L.. Isolation and structural indentification of herbicidal toxin fractions produced by Pythium aphanidermatum. Agric. Sci. China 9 (2010) 995–1000. DOI:10.1016/S1671-2927(09)60182-6 |
| [20] | Fan S.L., Zhang B., Gao L.Y., et al. Synthesis, antifungal activity and structureactivity relationship of 2-methoxycarbonyl/ethoxycarbonyl-4-fluorophenyl-1, 5-benzothiazepines. Chem. J. Chin. Univ. 35 (2014) 2574–2583. |
| [21] | Kondratieva M.L., Pepeleva A.V., Belskaia N.P., et al. A new synthetic method for the 2H-. Tetrahedron 63 (2007) 3042–3048. DOI:10.1016/j.tet.2007.01.059 |
| [22] | Efimov I., Bakulev V., Beliaev N., et al. Reactions of b-azolylenamines with sulfonyl azides as an approach to N-unsubstituted 1, 2, 3-triazoles and ethene-1, 2-diamines. Eur. J. Org. Chem. 2014 (2014) 3684–3689. DOI:10.1002/ejoc.201402130 |
| [23] | T.V. Beryozkina, I.V. Efimov, W.M.F. Fabian, et al., Reactivity of 1, 2, 3-triazoles towards sulfonyl chlorides. a novel approach to 1-and 2-sulfonyl-4-azolyl-1, 2, 3-triazoles, Tetrahedron 71(2015) 6189-6195. |
| [24] | Su W.N., Lin T.P., Cheng K.M., et al. An efficient one-pot synthesis of N-(1, 3-diphenyl-1H-pyrazol-5-yl) amides. J. Heterocycl. Chem. 47 (2010) 831–837. DOI:10.1002/jhet.343 |
 2016, Vol. 27
2016, Vol. 27