b School of Chemical and Environmental Engineering, Hubei University for Nationalities, Enshi 445000, China
Methyl parathion (MP) is a kind of organophosphorus pesticides, which are widely used as persistent pesticides and nerve agents [1]. The significantly high toxicity of MP could result in severe health problems on the visual system, sensory function, and cognitive function in both animals and humans [2]. In the past few decades, various analytical methods have been established for the determination of MP including fluorescence [3], surface enhanced Raman scattering [4], gas chromatography mass spectroscopy [5], high-performance liquid chromatography [6], chemiluminescence [7], and electrochemical sensing [8, 9]. Among these, electrochemical sensing route is gaining much attention because of its high portability, sensitivity, efficiency and accuracy. Thus, constant efforts are being exerted to find better materials for fabricating.
Nanomaterial-based electrochemical electrodes have shown tremendous potential with their high sensitivity, selectivity, and signal to noise ratio, attributed to their high surface area and unique electrochemical nature [10-12]. Among the various metallic and non-metallic nanomaterials used for electrode fabrication, nanosilica has elicited research interest in recent years owing to its unique properties, such as high mechanical strength, chemical inertness, thermal stability, and negligible swelling in aqueous and non-aqueous solutions, together with porosity, large surface areas, and pore volumes, which allow better loading of reactive molecules per particle [13]. Moreover, the potential of these economic nanosilica for sensing purposes was discovered once they were produced by the sol-gel technique [14], which showcased improved response time and detection limit [15, 16]. However, this porous structured sol-gel synthesized nanosilica decreases electrochemical activity by poor conductivity, which is a clear motivation for combining them with a conducting base material to form a composite material.
Graphene has caused considerable concern for its excellent electrochemical and mechanism performance [17] and has been applied widely in the fields of nanocomposites [18, 19], catalyst supports [20], electronic devices [21], sensors [22], and energy storage in batteries and supercapacitors [23]. Numerous technologies, such as chemical vapor deposition [24], epitaxial growth [25], chemical oxidation exfoliation [26, 27], and liquid-phase exfoliation [28], have been developed to prepare graphene sheets. Among these methods, liquid-phase exfoliation has attracted increasing attention due to its easy and gentle preparation process [29, 30]. Moreover, it has been proven that exfoliated graphene exhibits superior electrochemical activities over that of graphene oxides (GO) and reduced graphene oxides (RGO) [31, 32]. Furthermore, exfoliated graphene provides high surface-to-volume ratio and faster electron-transfer rate. Thus, the integration of nanosilica and exfoliated graphene could be expected to not only significantly improve the electrocatalytic properties of MP but also enhance the stability.
In this work, graphene nanosheets (GS) were easily prepared through short-time mild sonication of natural graphite powder in low-toxic N, N-dimethylformamide (DMF) based on sodium citrate assistance. The resulting GS suspension was then directly used to modify the surface of glassy carbon electrode. Subsequently, nanosilica was electrodeposited on the surface of the GS-modified GCE (GS/GCE), constructing a nanosilica and GS composite filmmodified GCE (nanosilica/GS/GCE). The oxidation behavior of MP was examined and an independent oxidation wave was observed. Compared with the bare GCE, the GS/GCE and the nanosilicamodified GCE (nanosilica/GCE) obviously increased the oxidation peak currents of MP. Interestingly, the nanosilica and GS exhibited a synergetic effect on the redox reaction of MP, and presented a significant enhancement on the current responses of MP. As a result, an effective electrochemical method with high sensitivity, selectivity and stability was developed for the determination of MP, and used in real samples analysis.
2. Experimental 2.1. Reagents and chemicalsAll of the chemicals were of analytical grade and used as received. Tetraethylorthosilicate was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). MP was purchased from Sanonda Corporation (Shashi, China) and the stock solution at 0.01 mol/L was prepared with ethanol and stored at 4 ℃. Test solutions were prepared daily by dilution of the stock solution with phosphate buffer solution. Phosphate buffer solutions were prepared from KH2PO4 and Na2HPO4. Ultrapure water was obtained from a Milli-Q water purification system.
2.2. InstrumentsElectrochemical measurements were performed on a CHI 660E electrochemical workstation (Chenhua Instrument, Shanghai, China). A glassy carbon electrode (GCE), a saturated calomel electrode (SCE), and a Pt wire were used for the working, reference, and counter electrodes, respectively. Scanning electron microscopic (SEM) measurements were conducted with a Quanta 200 microscope (FEI Company, The Netherlands). Atomic force microscopy (AFM) was performed with a SPM 9700 microscope (Shimadzu, Japan). Raman spectra were recorded using a RM-1000 confocal Raman microscopy system with a 514.5 nm laser (Renishaw Plc., Gloucestershire, UK). X-ray powder diffraction (XRD) was performed on a Bruker D8 X-ray diffractometer (Bruker, Germany) with Cu Kα radiation. X-ray photoelectron spectroscopy (XPS) was performed on VG MultiLab 2000 system (Thermo VG Scientific, UK).
2.3. Preparation of GS suspensionThe graphene suspension was prepared by sonicating the mixtures of graphite powder and DMF solvent with the addition of sodium citrate according to the modified procedures [33]. In a typical method, 30 mg of graphite powder and 10 mg of sodium citrate were added into 10 mL of DMF, and then sonicated in a KQ-2200 DE ultrasonicator (frequency: 50 kHz, power: 220 W; Kunshan Ultrasonic Instrument Co., Ltd, China) for 3.5 h at room temperature. Thus, 3.0 mg/mL suspension of GS was obtained.
2.4. Electrode modificationA solution containing 3.0 mL of 0.2 mol/L KCl, 2.0 mL of ethanol, and 0.50 mL of tetraethylorthosilicate was sonicated for 50 min until a transparent sol was observed. A GCE with a diameter of 3 mm was polished with 0.05 mm alumina slurry and then rinsed and sonicated with HNO3 (1:1), ethanol, and water for 3 min. Firstly, 5 μL of the resulting graphene suspension was coated on GCE surface, and then dried at 45 ℃ in the oven. After that, the electrodeposition of nanosilica on the GS/GCE was carried out in the pretreated sol with the constant potential at -2.0 V for 20 s. The nanosilica/GS/GCE was washed with ethanol and ultrapure water successively before taking measurement. Finally, the resulting nanocomposite showed strong signal enhancement effects for the detection of MP.
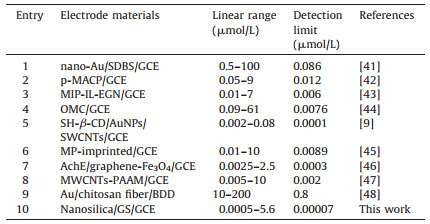
2.5. Analytical procedureUnless otherwise stated, 0.1 mol/L phosphate buffer solution at pH 7.0 was used as supporting electrolyte for MP detection. After 120-s accumulation at 0 V, the square wave voltammetries (SWVs) were recorded from -0.8 to 0.4 V, and the oxidation peak current at -0.12 V was used for MP determination. The amplitude was 25 mV, and frequency was 15 Hz.
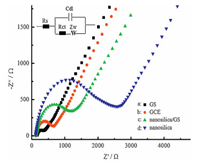
3. Results and discussionThe surface morphologies of the different modified GCEs were characterized using SEM. The surface of GS/GCE was coated by flaky sheets, indicating that graphite was exfoliated into graphene nanosheets (Fig. 1a). Sodium citrate acted as an exfoliating agent, which can markedly improve exfoliation efficiency and high-concentration graphene dispersion close to 3 mg/mL was easily obtained after only 3.5 h mild sonication. Fig. 1b shows that the electrode surface was obviously covered with a porous reticulated nanosilica film, which was efficiently synthesized using in situ electrodeposition method. These reticulations and nano-cavities significantly enhance the rebinding rate of MP. AFM images reveal that nanosilica/GS/GCE (Fig. 1d) had a much rougher surface compared with GS/GCE (Fig. 1c). Therefore, the nanosilica/GS/GCE displayed higher surface roughness, which could lead to larger effective sensing area and provide more active sites. Fig. 2a shows Raman spectra of the starting graphite powder and graphene sheets obtained from GS-DMF-sodium citrate system. The disorder-related D peak at 1354.9 cm-1 could be ascribed to edge defects introduced during sonication [34]. The sharp feature of the G peak (1583.9 cm-1) which was often observed for defective chemically converted graphene [35] indicates that the D band came from edges rather than structural defects inside graphene planes. The 2D band of graphene shifted to a lower position accompanying with an intensity increase compared to graphite. These features indicate that graphite was exfoliated into single-or few-layer graphene [36]. The Raman spectra of nanosilica and nanosilica/GS are shown in Fig. 2b. The Si-O vibration was observed around 1000 cm-1 which played a crucial role in empowering the nanosilica for the enhanced adsorptive and catalytic properties by providing links enabling enhanced charge transfer while maintaining better physical stability [37]. XRD spectra were used to analyze the interlayer spacing d 0 0 2 determined from the (0 0 2) diffraction peak position. Fig. 3a shows the XRD (0 0 2) peaks of raw graphite powders, and the GS with addition of sodium citrate. In these XRD patterns, the reflection peaks of the sample (b) was gradually shifted to a lower diffraction angle compared with the pattern of the sample (a), which indicates an increase in the interplanar spacing determined from Bragg’s law (2dsin θ=nλ) [38]. Although the interlayer spacing of graphite was increased to a limited extent, the increased interlayer spacing was favorable for the auxiliary agents (sodium citrate and solvent) to enter into the lattice of graphite, thus facilitating the exfoliation of graphite for the production of the graphene nanosheets. The XRD results in Fig. 3a indicate that sodium citrate intercalated into interplanar spaces of graphite. The XRD spectrum of the nanosilica/GS film on the GCE, shown in Fig. 3b, gives a weak broadening band between 20° and 25°, which indicated the presence of amorphous SiO2 [39]. XPS was used to characterize the change of composition and structure after anodization. The detailed processes are depicted in Supporting information (Fig. S1). The conductivity of the nanosilica/GS was measured by electrochemical impedance spectroscopy (EIS). Fig. 4 displays the EIS results of different electrodes, which were fitted based on the equivalent circuit as given in inset of Fig. 4. This equivalent circuit included the ohmic resistance of the electrolyte (Rs), the Warburg impedance (Zw), the electrontransfer resistance (Rct), and an interfacial capacitance (Cdl). The EIS included a semicircular part and a linear part. The semicircular part at higher frequency corresponded to the electron-transfer limited process, and the diameter was equivalent to the Rct. The results show that the Rct for the GS/GCE was 170 Ω, which was lower than the Rct values obtained for the bare GCE (500 Ω). This low Rct value for the GS modified electrode implied that the charge transfer process was relatively faster than the bare GCE. Compared to the impedance spectrum of nanosilica/GS (c) with that of nanosilica (d), the electron-transfer resistance (Rct) of nanosilica/GS was 1400 Ω, which was much lower than that of nanosilica (3600 Ω), demonstrating that the conductivity of nanosilica/GS was greatly enhanced.

|
Download:
|
| Figure 1. SEM (a and b) and AFM (c and d) images of GS/GCE (a and c), nanosilica/GS/GCE (b and d). | |

|
Download:
|
| Figure 2. (a) Raman spectra of graphite and GS, and (b) Raman spectra of nanosilica and nanosilica/GS. | |

|
Download:
|
| Figure 3. (a) The XRD patterns of raw graphite powders (a) and GS with addition of sodium citrate (b). The inset is a magnification of the green dashed rectangle area. (b) The XRD patterns of nanosilica/GS. | |
|
Download:
|
| Figure 4. Nyquist plot of GS (a), GCE (b), nanosilica/GS/GCE (c), nanosilica/GCE (d) in 5.0 × 10-3 mol/L [Fe(CN)6]3-/4- solution containing 0.1 mol/L KCl. | |
Apparently, graphene nanosheets provide high surface-tovolume ratio, faster electron-transfer and high conductivity. Meanwhile, nanosilica possesses high mechanical strength, tunable porosity, chemical inertness and thermal stability. In this work, the integration of the nanosilica and GS would not only significantly improve the electrocatalytic properties of MP but also enhance the stability.
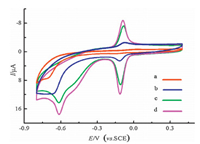
The electrochemical behaviors of MP on different GCEs were studied using cyclic voltammetry (CV), and the results are shown in Fig. 5. On the surface of the bare GCE (curve a), no obvious redox peaks were observed, suggesting that the electrochemical activity of MP was very low. However, a pair of rather well-defined redox peaks (-0.11 V and -0.09 V) and an irreversible reduction peak (-0.62 V) were observed for MP on the surfaces of nanosilica/GCE (curve b), GS/GCE (curve c), and nanosilica/GS/GCE (curve d) in the potential range from 0.4 V to -0.8 V. The appearance of sensitive redox waves, the irreversible reduction peak, and the greatly enhanced peak currents indicated that the prepared modified GCEs, especially the nanosilica/GS/GCE, obviously improved the electrochemical activity of MP. A similar electrochemical behavior was also observed for methyl parathion [40]. The electrochemical reactions of MP can be described as following:

|
|
Download:
|
| Figure 5. CV curves of 4 μmol/L MP in pH 7.0 phosphate buffer solution on GCE (curve a), nanosilica/GCE (curve b), GS/GCE (curve c), and nanosilica/GS/GCE (curve d). | |
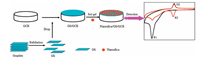
From Fig. 5, we found that only an irreversible reduction peak at -0.69 V was observed on the unmodified GCE (curve c). Compared with the unmodified GCE, the nanosilica/GCE, GS/GCE, and nanosilica/GS/GCE not only lowered the reduction overpotential of R1 but also remarkably increased the peak currents, especially the peak currents of O2 and R2. Thus, the nanosilica/GS/GCE possessed considerable surface enhancement effects and was more sensitive to the detection of MP (Scheme 1). The electrochemical behaviors of MP on different GCEs were studied using square wave voltammetry (SWV) and the results are shown in Fig. S2 in Supporting information.
|
Download:
|
| Scheme. 1. The fabricating procedures of nanosilica/GS/GCE and the electrochemical sensing mechanism of methyl parathion (MP). | |
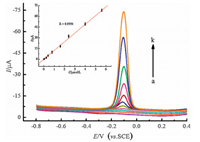
The effect of pH on the catalytic oxidation behavior was investigated to optimize the electrochemical response of nanosilica/GS/GCE toward MP oxidation. The SWVs of nanosilica/GS/GCE in 4 μmol/L MP at different pH values (4.9-8.0) were recorded. The oxidation peak currents of MP gradually increased with the pH values (4.9-7.0). However, the peak currents slightly decreased at pH > 7.0. A maximum value was obtained at pH of 7.0. The processes are showed in Fig. S3 in Supporting information. Meanwhile, effects of the accumulation potential and time on the SWV response for MP on nano-SiO2/GS/GCE were investigated. The detailed processes are depicted in Fig. S4. The SWVs of the modified electrode under optimal conditions at different concentrations of MP (0.0005 -5.6 μmol/L) were recorded (Fig. 6). The regression equation was Ip=-0.0795 + 12.3352c (Ip in μA, c in μmol/L, and R=0.9991) for MP (S/N=3). The electrochemical performance of the as-prepared film electrode in detecting MP was compared with previous works, and the merits of the analytical characteristics are shown in Table 1.
|
|
Table 1 Comparison of different electrodes for MP determination. |
|
Download:
|
| Figure 6. SWV curves of nanosilica/GS/GCE in pH 7.0 buffer containing different concentrations of MP (a k: 0, 0.0005, 0.007, 0.08, 0.24, 0.40, 0.80, 1.6, 2.4, 4.0, 5.6 μmol/L). The inset is the calibration curve for methyl parathion determination. | |
The reproducibility was evaluated by the nine successive measurements of the MP solution (3.0 μmol/L) with the same nanosilica/GS/GCE. A relative standard deviation (RSD) of 1.1% is obtained. The fabrication reproducibility was also investigated through the determination of the MP solution (3.0 μmol/L) with nine fresh nanosilica/GS/GCEs. The RSD obtained is 1.82%. Moreover, the nanosilica/GS/GCE showed no obvious decline even reused for 50 times (SD < 4%). This result indicates that the fabrication process is reliable and reproducible.
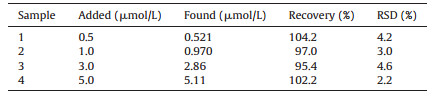
MP in the real samples was tested with the proposed film-modified electrode using the calibration method to demonstrate the applicability of the presented method. The results are listed in Table 2. The recoveries were in the range of 95.4%-104.2%, which indicated the applicability and reliability of the method. The selectivity is always a challenging task in electrochemical sensing fields. Thus, other pesticides and some inorganic ions which commonly coexisted in the real samples were also tested by using the developed nanosilica/GS/GCE. As shown in Fig. S5 in Supporting information, the results indicated that nanosilica/GS/ GCE possesses good selectivity towards MP.
|
|
Table 2 Determination of MP in real samples. |
4. Conclusion
Graphene nanosheets were easily obtained via solvent exfoliation of graphite powder assisted with organic salt, which could markedly improve exfoliation efficiency. The nanosilica/GS composite film-modified electrode was conveniently fabricated by drop and electrodeposition method. The resulting electrode demonstrated synergetic signal enhancement toward the oxidation of MP. Using nanosilica/GS composite film as the sensing material, a new electrochemical sensor was successfully constructed for MP detection. This proposed method possesses high sensitivity, selectivity, and stability, and was easily used for MP detection.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21561011), Scientific and Technological Innovation Team Project of Hubei University for Nationalities (No. MY2014T004), and the Open Foundation of Key Laboratory of Biologic Resources Protection and Utilization of Hubei Province (No. PKLHB1506).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.07.007.
| [1] | Manzanilla-Cano J.A., Reyes-Salas E.O., Barceló-Quintal M.H.. Electrochemical elimination of the pesticide methylparathion in an aqueous medium. Int. J. Environ. Anal. Chem. 75 (1999) 387–405. DOI:10.1080/03067319908047325 |
| [2] | M.B.Čolović, Krstić D.Z., Vasić V.M., et al. Organophosphorus insecticides:toxic effects and bioanalytical tests for evaluating toxicity during degradation processes. Hem. Ind. 67 (2013) 217–230. DOI:10.2298/HEMIND120323060C |
| [3] | Hou J., Dong J., Zhu H., et al. A simple and sensitive fluorescent sensor for methyl parathion based on L-tyrosine methyl ester functionalized carbon dots. Biosens. Bioelectron. 68 (2015) 20–26. DOI:10.1016/j.bios.2014.12.037 |
| [4] | Q. Zhou, G. Meng, N. Wu, et al., Dipping into a drink:Basil-seed supported silver nanoparticles as surface-enhanced Raman scattering substrates for toxic molecule detection, Sensor. Actuat. B-Chem. 233(2016), 477-452. |
| [5] | Cinelli G., Avino P., Notardonato I., et al. Ultrasound-vortex-assisted dispersive liquid-liquid microextraction coupled with gas chromatography with a nitrogen-phosphorus detector for simultaneous and rapid determination of organophosphorus pesticides and triazines in wine. Anal. Methods 6 (2014) 782–790. DOI:10.1039/C3AY41641K |
| [6] | Zhang Y., Lee H.K.. Determination of ultraviolet filters in environmental water samples by temperature-controlled ionic liquid dispersive liquid-phase microextraction. J. Chromatogr. A 1271 (2013) 56–61. DOI:10.1016/j.chroma.2012.11.047 |
| [7] | Zhang J., Wang J., Yang L., et al. Ligand replacement induced chemiluminescence for selective detection of an organophosphorus pesticide using bifunctional AuFe3O4 dumbbell-like nanoparticles. Chem. Commun. 50 (2014) 15870–15873. DOI:10.1039/C4CC07430K |
| [8] | Thota R., Ganesh V.. Selective and sensitive electrochemical detection of methyl parathion using chemically modified overhead projector sheets as flexible electrodes. Sensor. Actuat. B-Chem. 277 (2016) 169–177. |
| [9] | Fu X.C., Zhang J., Tao Y.Y., et al. Three-dimensional mono-6-thio-β-cyclodextrin covalently functionalized gold nanoparticle/single-wall carbon nanotube hybrids for highly sensitive and selective electrochemical determination of methyl parathion. Electrochim. Acta 153 (2015) 12–18. DOI:10.1016/j.electacta.2014.11.144 |
| [10] | Walcarius A., Minteer S.D., Wang J., et al. Nanomaterials for bio-functionalized electrodes:recent trends. J. Mater. Chem. B 1 (2013) 4878–4908. DOI:10.1039/c3tb20881h |
| [11] | Lu X.B., Wen Z.H., Li J.H.. Hydroxyl-containing antimony oxide bromide nanorods combined with chitosan for biosensors. Biomaterials 27 (2006) 5740–5747. DOI:10.1016/j.biomaterials.2006.07.026 |
| [12] | Abraham S., Nirala N.R., Pandey S., et al. Functional graphene-gold nanoparticle hybrid system for enhanced electrochemical biosensing of free cholesterol. Anal. Methods 7 (2015) 3993–4002. DOI:10.1039/C5AY00050E |
| [13] | Vida Y., Montañez M.I., Collado D., et al. Dendrimeric antigen-silica particle composites:an innovative approach for IgE quantification. J. Mater. Chem. B 1 (2013) 3044–3050. DOI:10.1039/c3tb20548g |
| [14] | Dave B.C., Dunn B., Valentine J.S., et al. Sol-gel encapsulation methods for biosensors. Anal. Chem. 66 (1994) 1120–1127. DOI:10.1021/ac00094a001 |
| [15] | Yang H., Zhu Y.. Size dependence of SiO2 particles enhanced glucose biosensor. Talanta 68 (2006) 569–574. DOI:10.1016/j.talanta.2005.04.057 |
| [16] | Dai Z., Liu S., Ju H., et al. Direct electron transfer and enzymatic activity of hemoglobin in a hexagonal mesoporous silica matrix. Biosens. Bioelectron. 19 (2004) 861–867. DOI:10.1016/j.bios.2003.08.024 |
| [17] | Tang L.H., Wang Y., Li Y.M., et al. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 19 (2009) 2782–2789. DOI:10.1002/adfm.v19:17 |
| [18] | Zhang Q., Zhang D., Lu Y., et al. Label-free amino acid detection based on nanocomposites of graphene oxide hybridized with gold nanoparticles. Biosens. Bioelectron. 77 (2016) 963–970. DOI:10.1016/j.bios.2015.10.065 |
| [19] | Chen Y., Dou X.W., Zhang M.M., et al. The fabrication of flower-like graphene/octadecylamine composites. Chin. Chem. Lett. 26 (2015) 1144–1146. DOI:10.1016/j.cclet.2015.05.045 |
| [20] | Mirabedini M., Motamedi E., Kassaee M.Z.. Magnetic CuO nanoparticles supported on graphene oxide as an efficient catalyst for A3-coupling synthesis of propargylamines. Chin. Chem. Lett. 26 (2015) 1085–1090. DOI:10.1016/j.cclet.2015.05.021 |
| [21] | Y. Jang, H. Jeong, D. Kim, et al., Electrical characterization of benzenedithiolate molecular electronic devices with graphene electrodes on rigid and flexible substrates, Nanotechnology 27(2016) 145301. |
| [22] | Chen D., Feng H.B., Li J.H.. Graphene oxide:preparation, functionalization, and electrochemical applications. Chem. Rev. 112 (2012) 6027–6053. DOI:10.1021/cr300115g |
| [23] | Zhang L.B., Yang S.R., Wang J.Q., et al. A facile preparation and electrochemical properties of nickel based compound-graphene sheet composites for supercapacitors. Chin. Chem. Lett. 26 (2015) 522–528. DOI:10.1016/j.cclet.2015.01.025 |
| [24] | Keeley G.P., McEvoy N., Nolan H.. Electroanalytical sensing properties of pristine and functionalized multilayer graphene. Chem. Mater. 26 (2014) 1807–1812. DOI:10.1021/cm403501r |
| [25] | Yang W., Chen G., Shi Z., et al. Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat. Mater. 12 (2013) 792–797. DOI:10.1038/nmat3695 |
| [26] | Wang Y., Lu J., Tang L.H., et al. Graphene oxide amplified electrogenerated chemiluminescence of quantum dots and its selective sensing for glutathione from thiol-containing compounds. Anal. Chem. 81 (2009) 9710–9715. DOI:10.1021/ac901935a |
| [27] | Sun J.J., Yang N.X., Sun Z., et al. Fully converting graphite into graphene oxide hydrogels by preoxidation with impure manganese dioxide. ACS Appl. Mater. Inter. 7 (2015) 21356–21363. DOI:10.1021/acsami.5b06008 |
| [28] | Xu M., Zhang W., Yang Z., et al. One-pot liquid-phase exfoliation from graphite to graphene with carbon quantum dots. Nanoscale 7 (2015) 10527–10534. DOI:10.1039/C5NR02198G |
| [29] | Ciesielski A., Samorı P.. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 43 (2014) 381–398. DOI:10.1039/C3CS60217F |
| [30] | Wu C., Tang Y., Wan C., et al. Enhanced-oxidation and highly-sensitive detection of acetaminophen, guanine and adenine using NMP-exfoliated graphene nanosheets-modified electrode. Electrochim. Acta 166 (2015) 285–292. DOI:10.1016/j.electacta.2015.03.088 |
| [31] | Wu C., Cheng Q., Wu K.. Electrochemical functionalization of N-methyl-2-pyrrolidone-exfoliated graphene nanosheets as highly sensitive analytical platform for phenols. Anal. Chem. 87 (2015) 3294–3299. DOI:10.1021/ac504309j |
| [32] | Yang X., Long J., Sun D.. Highly-sensitive electrochemical determination of rutin using NMP-exfoliated graphene nanosheets-modified electrode. Electroanalysis 28 (2016) 83–87. DOI:10.1002/elan.v28.1 |
| [33] | Du W.C., Lu J., Sun P.P., et al. Organic salt-assisted liquid-phase exfoliation of graphite to produce high-quality graphene. Chem. Phys. Lett. 568- 569 (2013) 198–201. |
| [34] | De S., King P.J., Lotya M., et al. Flexible, transparent, conducting films of randomly stacked graphene from surfactant-stabilized, oxide-free graphene dispersions. Small 6 (2010) 458–464. DOI:10.1002/smll.v6:3 |
| [35] | Kudin K.N., Ozbas B., Schniepp H.C., et al. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8 (2008) 36–41. DOI:10.1021/nl071822y |
| [36] | Allen M.J., Tung V.C., Kaner R.B.. Honeycomb carbon a review of graphene. Chem. Rev. 110 (2010) 132–145. DOI:10.1021/cr900070d |
| [37] | Bermudez V.M.. Effect of humidity on the interaction of dimethyl methylphosphonate (DMMP) vapor with SiO2 and Al2O3 surfaces, studied using infrared attenuated total reflection spectroscopy. Langmuir 26 (2010) 18144–18154. DOI:10.1021/la103381r |
| [38] | Xu J., Zhang J., Liu X.. Hydrothermal synthesis of copper hydroxyphosphate hierarchical architectures. Chem. Eng. Technol. 35 (2012) 2189–2194. DOI:10.1002/ceat.v35.12 |
| [39] | N. Yan, F. Wang, H. Zhong, et al., Hollow porous SiO2 nanocubes towards highperformance anodes for lithium-ion batteries, Sci. Rep.-UK 3(2013) 1568. |
| [40] | Liu G.D., Lin Y.H.. Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal. Chem. 77 (2005) 5894–5901. DOI:10.1021/ac050791t |
| [41] | Li C., Wang Z., Zhan G.. Electrochemical investigation of methyl parathion at goldsodium dodecylbenzene sulfonate nanoparticles modified glassy carbon electrode. Colloids Surf. B-Biointerfaces 82 (2011) 40–45. DOI:10.1016/j.colsurfb.2010.08.011 |
| [42] | Tan X.H., Zhang S.H., Song X.J., et al. A nanostructured poly(2-mercapto-4-amino-5-cyano-6-phenylpyrimidine) film for sensitive determination of methyl parathion. Nanosci. Nanotechnol. Lett. 6 (2014) 333–338. DOI:10.1166/nnl.2014.1763 |
| [43] | Zhao L., Zhao F., Zeng B.. Electrochemical determination of methyl parathion using a molecularly imprinted polymer-ionic liquid-graphene composite film coated electrode. Sensor. Actuat. B-Chem. 176 (2013) 818–824. DOI:10.1016/j.snb.2012.10.003 |
| [44] | Pan D., Ma S., Bo X., et al. Electrochemical behavior of methyl parathion and its sensitive determination at a glassy carbon electrode modified with ordered mesoporous carbon. Microchim. Acta 173 (2011) 215–221. DOI:10.1007/s00604-011-0551-1 |
| [45] | Tan X., Li B., Liew K.Y.. Electrochemical fabrication of molecularly imprinted porous silicate film electrode for fast and selective response of methyl parathion. Biosens. Bioelectron. 26 (2010) 868–871. DOI:10.1016/j.bios.2010.07.085 |
| [46] | Liu Z.M., Jing Y.F., Wang Z.L., et al. Highly sensitive electrochemical biosensing of methyl parathion pesticide based on acetylcholinesterase immobilized onto graphene-Fe3O4 nanocomposite. Sens. Lett. 11 (2013) 531–538. DOI:10.1166/sl.2013.2762 |
| [47] | Zeng Y., Yu D., Yu Y., et al. Differential pulse voltammetric determination of methyl parathion based on multiwalled carbon nanotubes-poly (acrylamide) nanocomposite film modified electrode. J. Hazard. Mater. 217 (2012) 315–322. |
| [48] | Jin H., Liu Y., Wei M.. Determination of organophosphorus pesticides based on BDD electrode modified with Au/chitosan fiber. J. Chin. Chem. Soc. 60 (2013) 297–302. DOI:10.1002/jccs.v60.3 |
 2016, Vol. 27
2016, Vol. 27