Because of the stability of an aromatic ring, the exocyclic double bond in styrene commonly reacts as a dienophile in [4 + 2] cycloaddition reactions[1] and it is quite difficult for styrene to participate as a diene in thermal Diels-Alder cycloaddition unless extremely reactive dienophiles are employed[2]. 2H-pyran-2-one and its derivatives are a class of important electron-rich diene in the Diels-Alder reaction[3] and are widely used in organic synthesis[4]. There is a feature of this type of dienes in the Diels-Alder reaction: Retro-Diels-Alder reaction will happen under certain conditions by eliminating one molecule of CO2, which introduces a carbon-carbon double bond in the ring[5]. Several reports claimed that 2H-chromen-2-one, a kind of 2H-pyran-2-one fused with a benzene ring, can be used in the Diels-Alder cycloaddition as a dienophile[6]. However, only one case where it was used as a diene was reported in the tandem intramolecular Diels-Alder (IMDA) and retro-Diels-Alder cycloaddition with acetylene as shown in Scheme 1[7]. The reaction must be carried out in a sealed tube placed in a 300 ℃ bath, and surprisingly, the acetylenic hydrogen replaced by an electron-withdrawing group (EWG), carboethoxy, 1e failed to cyclize to form 2e. In our continuous works on synthesis of polycyclic compounds using IMDA reaction[8], herein, we report the tandem IMDA and retro-Diels-Alder reaction of chiral N-(4-methoxybenzyl)-2-oxo-N-(1-phenylbut-3-en-2-yl)-2H-chromene-3-carboxamides for stereocontrolled synthesis of 3-benzyl-2-(4-methoxybenzyl)-2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindol-1-ones under relatively mild conditions.

|
Download:
|
| Scheme. 1. Tandem IMDA and retro-Diels–Alder reaction of 2H-chromen-2-one with acetylene. | |
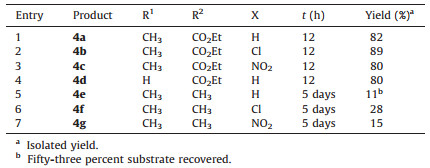
2. Experimental 2.1. General procedure for the synthesis of 4a-g
A solution of 3 (0.1 mmol) in CH3CN (2 mL) in a sealed vial was heated in an oil bath at 160 ℃. After the reaction completed (monitored by TLC analysis), the solvent was removed under reduced pressure. The residue was purified by chromatography on silica gel (ethyl acetate/petroleum ether=1/3) to give 4 (Scheme 2).
|
Download:
|
| Scheme. 2. Synthesis of compounds 4a–g. | |
Ethyl (3S, 3aS, 4R)-3-benzyl-2-(4-methoxybenzyl)-4-methyl-1-oxo-2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindole-4-carboxylate 4a: White crystalline solid, yield 82%, mp 204-205 ℃ (EtOAc-hexane); [α]D25 +13.9 (c 1.0, CHCl3); Rf=0.20 (20% EtOAc in hexane); IR (film, cm-1): 3061, 3028, 2979, 2933, 2836, 1721, 1685, 1611, 1512, 1447, 1409, 1298, 1247, 1174, 1093, 1031, 909, 755, 731, 700; 1H NMR (400 MHz, CDCl3): δ 7.29-7.21 (m, 6H), 7.19 (d, 1H, J=3.2 Hz), 7.11-7.09 (m, 2H), 6.94-6.90 (m, 3H), 6.78 (d, 2H, J=8.8 Hz), 5.34 and 3.91 (ABq, 2H, J=14.8 Hz), 4.32 (dq, 1H, J =10.8, 7.2 Hz), 4.19 (dq, 1H, J=10.8, 7.2 Hz), 3.79 (s, 3H), 3.76 (dt, 1H, J=5.2, 3.6 Hz), 3.65 (dd, 1H, J=4.8, 3.2 Hz), 3.12 (dd, 1H, J=15.2, 3.2 Hz), 2.66 (dd, 1H, J=15.2, 5.6 Hz), 1.31 (t, 3H, J=7.2 Hz), 1.11 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 175.8, 167.0, 159.0, 139.6, 136.4, 132.2, 131.6, 129.7, 129.6 (×2), 129.2 (×2), 129.1, 128.6 (×2), 127.9, 127.7, 126.8, 126.0, 125.4, 114.0 (×2), 61.6, 55.6, 55.2, 51.2, 44.2, 42.9, 38.2, 17.2, 14.2; HRMS (+EI) calculated for C31H31NO4+ (M+) 481.2253; found 481.2246.
(3S, 3aR)-3-Benzyl-2-(4-methoxybenzyl)-4, 4-dimethyl-2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindol-1-one 4e: Colorless oil, yield 11%, [α]D25 +7.6 (c 1.0, CHCl3); Rf=0.19 (25% EtOAc in hexane); IR (film, cm-1): 3066, 3030, 3003, 2965, 2929, 2866, 2834, 1676, 1611, 1516, 1453, 1409, 1304, 1239, 1174, 1031, 834, 811, 751, 703; 1H NMR (400 MHz, CDCl3): δ 7.27-7.19 (m, 8H), 7.09-7.04 (m, 4H), 6.85 (d, 2H, J=8.8 Hz), 5.40and 4.01(ABq, 2H, J=14.8 Hz), 3.81(s, 3H), 3.67-3.63 (m, 1H), 3.02-2.92 (m, 2H), 2.78-2.75 (m, 1H), 1.03 (s, 3H), 0.74 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 167.4, 159.1, 144.8, 136.6, 133.7, 132.5, 129.8 (×2), 129.5, 129.4 (×2), 128.9, 128.7 (×2), 128.1, 126.9, 126.8, 126.7, 123.6, 114.0 (×2), 56.2, 55.3, 46.0, 44.1, 40.1, 37.5, 24.3, 21.6; HRMS (+EI) calculated for C29H29NO2+ (M+) 423.2198; found 423.2204.
2.2. General procedure for the synthesis of 5a-cTo a solution of 4 (0.1 mmol) in a mixed solvent of 2-methyl-2-propanol (1 mL) and water (1 mL) were added methanesulfonamide (0.1 mmol) and AD-mix α (145 mg). After stirring at 0 ℃ for 10-24 h, the reaction mixture was quenched with saturated aqueous Na2S2O3 (5 mL) and stirred at room temperature for another 20 min, then the mixture was extracted with ethyl acetate (10 mL × 3). The combined organic phase was dried over anhydrous Na2SO4, filtrated and concentrated under reduced pressure. The residue was purified by chromatography on silica gel (EtOAc/ PE=1/1) to give 5 (Scheme 3).

|
Download:
|
| Scheme. 3. Synthesis of compounds 5a–c. | |
(1S, 3aS, 4R, 9R, 9aS)-1-Benzyl-3a-hydroxy-2-(4-methoxybenzyl)-9-methyl-3a, 4, 9, 9a-tetrahydro-1H-4, 9-(epoxymethano)benzo[f]-isoindole-3, 10(2H)-dione 5a: White crystalline solid, yield 90%; mp 102-107 ℃ (EtOAc-hexane); [α]D25 -4.2 (c 1.0, CHCl3); Rf=0.22 (50%EtOAcinhexane); IR(film, cm-1):3387, 3063, 3033, 2980, 2938, 2840, 1757, 1676, 1513, 1453, 1373, 1245, 1043, 1004, 843, 778, 745, 701; 1H NMR (400 MHz, CDCl3): δ 7.48-7.46 (m, 1H), 7.41-7.34 (m, 2H), 7.33-7.25 (m, 3H), 6.99-6.94 (m, 3H), 6.67 (d, 2H, J=8.8 Hz), 6.23 (d, 2H, J=8.8 Hz), 5.64 (s, 1H), 4.78 and 3.72 (ABq, 2H, J=14.8 Hz), 3.83 (s, 3H), 3.15 (dd, 1H, J=13.2, 3.6 Hz), 2.73 (ddd, 1H, J=9.6, 3.6, 2.4 Hz), 2.55 (dd, 1H, J=13.2, 9.6 Hz), 2.34 (d, 1H, J=2.4 Hz), 0.76 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 173.3, 170.7, 159.0, 135.5, 135.3, 134.3, 129.8, 129.7 (×2), 129.4 (×2), 129.0 (×2), 128.1, 127.4, 125.4, 125.3, 123.9, 114.1 (×2), 82.1, 81.5, 58.1, 55.3, 47.8, 47.6, 43.8, 39.8, 12.6; HRMS (+EI) calculated for C29H27NO5+ (M+) 469.1889; found 469.1881.
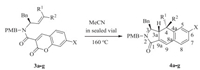
2.3. Synthesis of 6c[9]Some biological studies on the approved and developing γ-lactam drugs showed that the N-H type lactams are more active than the corresponding N-substituted one[11], therefore we completed the transformation of 4c to 6c as an example using ceric (Ⅳ) ammonium nitrate (CAN). To a solution of 4c (72 mg, 0.137 mmol) in a mixed solvent of CH3CN (3 mL) and H2O (1 mL) was added ceric ammonium nitrate (298 mg, 0.544 mmol) in one portion. After stirring for 30 min at room temperature, H2O (3 mL) was added and then the mixture was extracted with ethyl acetate (10 mL × 3). The combined organic layer was washed with saturated aqueous NaHCO3 (1.5 mL × 3) and brine (1.5 mL). The organic phase was dried over anhydrous Na2SO4, filtrated and concentrated under reduced pressure. The residue was purified by chromatography on silica gel (ethyl acetate/petroleum=1/1) to give 6c (46 mg, 83%) as a colorless oil (Scheme 4).
|
Download:
|
| Scheme. 4. Transformation from 4c to 6c by removing PMB with CAN. | |
Ethyl (3S)-3-benzyl-4-methyl-7-nitro-1-oxo-2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindole-4-carboxylate 6c: Yield 83%; [α]D15 +2.1 (c 1.0, CHCl3); Rf=0.29 (50% EtOAc in hexane); IR (film, cm-1): 3405, 3214, 3066, 3024, 2979, 2935, 2873, 1730, 1697, 1608, 1584, 1519, 1456, 1346, 1292, 1239, 1090, 1050, 1016, 909, 825, 736, 698; 1H NMR (400 MHz, CDCl3): δ 8.14-8.12 (m, 2H), 7.35 (t, 2H, J=7.2 Hz), 7.29 (d, 1H, J=7.2 Hz), 7.25 (d, 1H, J=3.2 Hz), 7.21 (d, 1H, J=7.2 Hz), 7.19 (d, 2H, J=8.0 Hz), 6.18 (br s, 1H), 4.47-4.34 (m, 2H), 3.98-3.93 (m, 1H), 3.70 (dd, 1H, J=6.0, 3.6 Hz), 2.98 (dd, 1H, J=13.6, 2.8 Hz), 2.60 (dd, 1H, J=13.6, 10.0 Hz), 1.39 (t, 3H, J=7.2 Hz), 1.38 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 174.7, 167.1, 147.3, 146.1, 136.4, 135.3, 132.8, 129.1 (×2), 129.0 (×2), 127.3, 126.7, 124.32, 124.29, 123.4, 62.3, 54.6, 51.0, 46.4, 42.0, 17.8, 14.2; HRMS (+EI) calcd. for C23H22N2O5+ (M+) 406.1529; found 406.1538.
3. Results and discussionWe designed and synthesized a series of chiral N-allyl-N-benzylamides containing the moiety of 2H-chromen-2-one (3a-g) for the IMDA reaction with the expectation that the 2H-chromen-2-one moiety and the allyl group act as the diene and the dienophile, respectively. The optimized reaction conditions are by heating a solution of chiral amide 3 inacetonitrile in a sealed vial placedin an oil bath at 160 ℃. The chiral N-(4-methoxybenzyl)-2-oxo-N-(1-phenylbut-3-en-2-yl)-2H-chromene-3-carboxamides 3a-g were transformed into tricyclic compounds, 3-benzyl-2-(4-methoxy-benzyl)-2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindol-1-ones 4a-g (entries 1-7, Table 1) via an IMDA followed by a retro-Diels-Alder reaction with the expulsion of CO2. The stereochemistry of these adducts was absolutely controlled by the preexisting chiral center.
|
|
Table 1 Results of tandem IMDA and retro-Diels–Alder reaction of 3. |
The reactivity of amides varied depending on the substitution pattern at the terminal of the allyl double bond. As shown in Table 1, those amides attached to a trans electron-withdrawing group, carboethoxy, at the terminal of the allyl double bond 3a-d reacted to furnish 4a-d within 12 h in high yields (80%-89%) (Table 1, entries 1-4). However, amides attached to two methyl groups at the terminal allyl double bond 3e-g were transformed into 4e-g in low yields (11%-28%) and much longer time (5 days) was needed (Table 1, entries 5-7). These results show that an electron-withdrawing group on the dienophiles facilitates the reaction but an electron-donating group is unfavorable. Interestingly, these results are completely different from that of the IMDA reaction of 2H-chromen-2-one with acetylenic groups. That reaction required higher temperature (300 ℃) and was retarded by an electron-withdrawing group, carboethoxy, attached to the terminal acetylene (Scheme 1). The substituents on the benzene ring of 2H-chromen-2-one do not apparently affect the reactivity (entries 1-3 and 5-7, Table 1), but weak electron-withdrawing groups on the benzene ring of 2H-chromen-2-one facilitate the reaction (entries 2 and 6, Table 1).
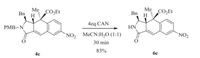
The structure of the product 4a, depicted in Fig. 1, was determined by X-ray diffraction analysis. The crystallographic data of 4a have been deposited in the Cambridge Crystallographic Data Centre (No. CCDC 1451810). The structures of 4b-g were deduced from 4a by comparing the 1H NMR data. The coupling constants between H3 and H3a of 4b-g are in a range of 3.6-5.6 Hz and similar to that of 4a (4.8 Hz). Paddon-Row and Sherburn[10] performed computational studies on the stereoselectivity in IMDA reactions of penta-1, 3-dienyl acrylates and found that trans adducts were generally favored. Accordingly, we hypothesized a possible pathway of cycloaddition of 3a as shown in Scheme 5. The chiral N-allyl-N-benzylamide transforms into IMDA adduct B via transition state A, and the subsequent elimination of carbon dioxide from B generates the 2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindol-1-one derivative 4a.
|
Download:
|
| Figure 1. X-ray structure for 4a. | |

|
Download:
|
| Scheme. 5. An assumed possible pathway from 3a to 4a. | |
Due to the C9-C9a double bond, these 2, 3, 3a, 4-tetrahydro-1H-benzo[f]isoindol-1-ones can be subjected to further modifications. Stereo-controlled dihydroxylation of 4a-c by AD-mix α followed by esterification afforded 5a-c in high yields (83%-90%) (Scheme 3). It is surprising to us that the formed products had a δ-lactone structure. These results show that the two introduced hydroxyl groups were kept in the same face of the carbon ring with carboethoxy, which led to the formation of the δ-lactone with the hydroxyl at the C9 position under basic conditions.
4. ConclusionThe tandem IMDA/retro-Diels-Alder reaction of chiral N-allyl-N-benzylamides containing 2H-chromen-2-one constitutes an absolute stereo-controlled entry to 2, 3, 3a, 4-tetrahydro-1H-ben-zo[f]isoindol-1-ones. The reactivity was affected by groups attached in the dienophile, and an electron-withdrawing group facilitates the reaction. Adducts can be further modified by removing the PMB group or dihydroxylation of the double bond. Further studies on the scope and application of this tandem IMDA/ retro-Diels-Alder reaction of N-allyl-N-benzylamides containing 2H-chromen-2-ones are in progress.
AcknowledgmentThis work was supported by the research grant from the National Natural Science Foundation of China (No. 21172191). Mr. Jiyong Liu of the X-ray crystallography facility of Zhejiang University is acknowledged for his assistance in the crystal structure analysis.
| [1] |
(a) J.S. Meek, R.T. Merrow, S.J. Cristol, The Diels-Alder reaction of isoprene with styrene and 2-vinylpyridine1, J. Am. Chem. Soc. 74(1952) 2667-2668; (b) T.G. Corbett, W. Davies, Q.N. Porter, Reactions of O-quinones with 1-vinylnaphthalene, Aust. J. Chem. 18(1965) 1775-1779; (c) D.R. Arnold, L.B. Gillis, E.B. Whipple, Photocycloaddition of 2-acetylnaphthalene to methyl cinnamate. A 2+4 electron photocycloaddition, J. Chem. Soc. D (1969) 918-919; (d) N.N. Podgornova, E.S. Lipina, T.Y. Paperno, V.V. Perekalin, 1, 4-dinitrodienes in a "reversed" Diels-Alder condensation reaction, Zhurnal Org. Khimii 12(1976) 25-32; (e) V.M. Berestovitskaya, M.V. Titova, V.V. Perekalin, Dinitro-3-sulfolenes as sources of dinitro-1, 3-butadienes in the Diels-Alder reaction with "inverse" electron properties, Zhurnal Org. Khimii 16(1980) 891-892; (f) D. Hartsough, G.B. Schuster, The triplex Diels-Alder reaction:stereospecific addition of methylstyrenes to 1, 3-cyclohexadiene, J. Org. Chem. 54(1989) 3-4; (g) V. Nair, D. Maliakal, P.M. Treesa, N.P. Rath, G.K. Eigendorf, [4+2] cycloaddition reactions of o-benzoquinones with styrenes:a facile synthesis of bicyclo[2.2.2] octenediones, Synthesis (2000) 850-856; (h) Y.S. Lin, S.Y. Chang, M.S. Yang, et al., Diels-Alder reactions of 4-triflyloxy-2, 6, 6-trimethyl-2, 4-cyclohexadienone. An expedient methodology for the synthesis of bicyclo[2.2.2] oct-5-en-2-ones and bicyclo[2.2.2] octa-5, 7-dien-2-ones, J. Org. Chem. 69(2004) 447-458. |
| [2] | Theodor W.J.. Thermische und photochemische additionen von dienophilen an arene sowie deren vinyloge und hetero-analoge; Ⅱ. Synthesis 1980 (1980) 769–798. DOI:10.1055/s-1980-29206 |
| [3] |
(a) J.A. Reed, C.L. Schilling Jr., R. Tarvin, T.A. Rettig, J.K. Stille, Diels-Alder reactions of 2-pyrones. Direction of the addition reaction with acetylenes, J. Org. Chem. 34(1969) 2188-2192; (b) G.H. Posner, T.D. Nelson, C.M. Kinter, K. Afarinkia, 3-Bromo-2-pyrone:an easily prepared chameleon diene and a synthetic equivalent of 2-pyrone in thermal Diels-Alder cycloadditions, Tetrahedron Lett. 32(1991) 5295-5298; (c) K. Afarinkia, G.H. Posner, 5-Bromo-2-pyrone:an easily prepared ambiphilic diene and a synthetic equivalent of 2-pyrone in mild, thermal Diels-Alder cycloadditions, Tetrahedron Lett. 33(1992) 7839-7842; (d) K. Afarinkia, B.C. Jose, Diels-Alder cycloaddition of 5-aryl-2-pyrones, Tetrahedron Lett. 41(2000) 4955-4958; (e) C.G. Cho, Y.W. Kim, Y.K. Lim, et al., Diels-Alder cycloadditions of 3, 5-dibromo-2-pyrone:a new ambident diene, J. Org. Chem. 67(2002) 290-293; (f) K. Afarinkia, M.J. Bearpark, A. Ndibwami, An experimental and computational investigation of the Diels-Alder cycloadditions of halogen-substituted 2(H)-pyran-2-ones, J. Org. Chem. 70(2005) 1122-1133. |
| [4] |
(a) D.A. Bleasdale, D.W. Jones, Regioselective Diels-Alder addition to 2-benzopyran-3-ones:a route to aromatic steroids, J. Chem. Soc. Chem. Commun. (1985) 1027-1028; (b) E.J. Bush, D.W. Jones, Asymmetric total synthesis of (±)-podophyllotoxin, J. Chem. Soc. Chem. Commun. (1993) 1200-1201; (c) I.J. Shin, E.S. Choi, C.G. Cho, Total synthesis of (±)-trans-dihydronarciclasine through a highly endo-selective Diels-Alder cycloaddition of 3, 5-dibromo-2-pyrone, Angew. Chem. Int. Ed. 119(2007) 2353-2355; (d) N.T. Tam, C.G. Cho, Total synthesis of (±)-joubertinamine from 3-(3, 4-dimethoxyphenyl)-5-bromo-2-pyrone, Org. Lett. 9(2007) 3391-3392; (e) M.V. Kozytska, G.B. Dudley, On the intramolecular pyrone Diels-Alder approach to basiliolide B, Tetrahedron Lett. 49(2008) 2899-2901; (f) N.T. Tam, J. Chang, E.J. Jung, C.G. Cho, Total syntheses of (±)-crinine, (±)-crinamine, and (±)-6a-epi-crinamine via the regioselective synthesis and Diels-Alder reaction of 3-aryl-5-bromo-2-pyrone, J. Org. Chem. 73(2008) 6258-6264; (g) N.T. Tam, C.G. Cho, Total synthesis of (±)-crinine via the regioselective stille coupling and Diels-Alder reaction of 3, 5-dibromo-2-pyrone, Org. Lett. 10(2008) 601-603; (h) T.T. Nguyen, E.J. Jung, C.G. Cho, Intramolecular imino Diels-Alder approach to the synthesis of the aspidosperma alkaloid from 3, 5-dibromo-2-pyrone, Org. Lett. 12(2010) 2012-2014; (i) Y.G. Jung, H.U. Kang, H.K. Cho, C.G. Cho, B-silyl styrene as a dienophile in the cycloaddition with 3, 5-dibromo-2-pyrone for the total synthesis of (±)-pancratistatin, Org. Lett. 13(2011) 5890-5892; (j) H.K. Cho, H.Y. Lim, C.G. Cho, (E)-b-Borylstyrene in the Diels-Alder reaction with 3, 5-dibromo-2-pyrone for the syntheses of (±)-1-epi-pancratistatin and (±)-pancratistatin, Org. Lett. 15(2013) 5806-5809; (k) Y.G. Jung, S.C. Lee, H.K. Cho, et al., Total syntheses of (±)-α-lycorane and (±)-1-deoxylycorine, Org. Lett. 15(2013) 132-135. |
| [5] |
(a) Leo A. Paquette, et al. Organic Reaction, Vol. 53, John Wiley & Sons, Inc, New York, 1998, pp. 223-629; (b) A. Ichihara, Retro-Diels-Alder strategy in natural product synthesis, Synthesis (1987) 207-222. |
| [6] |
(a) R. Adams, W.D. McPhee, R.B. Carlin, Z.W. Wicks, The addition of dienes to coumarin and to certain substituted cinnamic acids. I, J. Am. Chem. Soc. 65(1943) 356-360; (b) D. Amantini, F. Fringuelli, O. Piermatti, F. Pizzo, L. Vaccaro, 3-Nitrocoumarins as dienophiles in the Diels-Alder reaction in water. An approach to the synthesis of nitrotetrahydrobenzo[c]chromenones dihydrodibenzo-[b, d]furans, J. Org. Chem. 68(2003) 9263-9268; (c) R. Girotti, A. Marrocchi, L. Minuti, et al., Diels-Alder reaction of 3-substituted coumarins in water and under high-pressure condition. An uncatalyzed route to tetrahydro-6H-benzo[c]chromen-6-ones, J. Org. Chem. 71(2006) 70-74; (d) L. Minuti, A. Marrocchi, S. Landi, M. Seri, E. Gacs-Baitz, High-pressure and thermal Diels-Alder reactions of 5-nitro[2.2] paracyclophanepyran-6-one, Tetrahedron 63(2007) 5477-5481; (e) E. Balerini, L. Minuti, O. Piermatti, High pressure Diels-Alder approach to hydroxy substituted 6a-cyano tetrahydro-6H-benzo[c]chromen-6-ones:a route to D6-cis-cannabidiol, J. Org. Chem. 74(2009) 4311-4317; (f) I.Y. Flores-Rarios, L. Lopez-Garrido, F.J. Martinez-Martinez, et al., Thermal[4+2] cycloaddition of 3-acetyl, 3-carbamoyl and 3-ethoxycarbonyl-coumarins with 2, 3-dimethyl 1, 3-butadiene under solventless conditions:a structural study, Molecules 15(2010) 1513-1530; (g) A.G. Song, X.S. Zhang, X.X. Song, et al., Construction of chiral bridged tricyclic benzopyranes:enantioselective catalytic Diels-Alder reaction and a one-pot reduction/acid-catalyzed stereoselective cyclization, Angew. Chem. Int. Ed. 53(2014) 4940-4944. |
| [7] | G.A. Kraus, PezzaniteF J.O., H. Sugimoto, Hetreocyclic intraconversion, Tetrahedron Lett. (1979) 853-856. |
| [8] |
(a) J. Wu, L. Sun, W.M. Dai, Microwave-assisted tandem Wittig-intramolecular Diels-Alder cycloaddition. Product distribution and stereochemical assignment, Tetrahedron 62(2006) 8360-8372; (b) J. Wu, H. Yu, Y. Wang, X. Xing, W.M. Dai, Unexpected epimerization and stereochemistry revision of IMDA adducts from sorbate-related 1, 3, 8-nonatrienes, Tetrahedron Lett. 48(2007) 6543-6547; (c) J. Wu, X. Jiang, J. Xu, W.M. Dai, Tandem Wittig-intramolecular Diels-Alder cycloaddition of ester-tethered 1, 3, 9-decatrienes under microwave heating, Tetrahedron 67(2011) 179-192. |
| [9] | Huang P.Q., Wu T.J., Ruan Y.P.. A flexible approach to (s)-5-alkyl tetramic acid derivatives:application to the asymmetric synthesis of (+)-preussin and protected (3s, 4s)-ahppa. Org. Lett. 5 (2003) 4341–4344. DOI:10.1021/ol035617a |
| [10] |
(a) T.N. Cayzer, M.N. Paddon-Row, D. Moran, A.D. Payne, Intramolecular Diels-Alder reaction of ester-linked 1, 3, 8-nonatrienes, J. Org. Chem. 70(2005) 5561-5570; (b) M.N. Paddon-Row, D. Moran, G.A. Jones, M.S. Sherburn, On the origin of cis/trans stereoselectivity in intramolecular Diels-Alder reactions of substituted pentadienylacrylates:a comprehensive density functional study, J. Org. Chem. 70(2005) 10841-10853. |
| [11] | Tan D.Q., Martin K.S., Fettinger J.C., Shaw J.T.. Ammonia synthons for the multicomponent assembly of complex gamma-lactams. Proc. Natl. Acad. Sci. 108 (2011) 6781–6786. DOI:10.1073/pnas.1015261108 |
 2016, Vol. 27
2016, Vol. 27