The catalytic utilization of molecular iodine (I2) and its salts in organic synthesis is increasing in importance, with growing interest in the development of environmentally benign synthetic transformations [1]. Due to the ease of handling, low toxicity, commercial availability and mild reactivity, especially the metallike behavior of iodine, a number of recent studies have demonstrated the utility of iodine catalysis as an efficient and powerful tool for the formation of carbon-carbon and carbon-heteroatom bonds [2-11]. Lei and co-workers have reported an I2-catalyzed radical alkenylation of sulfonyl hydrazides, and various alkenyl sulfones have been synthesized [12]. Similarly, other methods for construction of carbon-sulfur bond using I2 as catalyst have also been developed from alkenes and sodium sulfinates [13-19]. More recently, the I2-mediated or I2-catalyzed synthesis of sulfonamides from sodium sulfinates and amines has been investigated [20-23], it is very interesting because this nitrogen-sulfur bond formation method is general, efficient and environmentally friendly. In addition, the given protocol provides good to excellent yields of sulfonamides under mild reaction conditions.
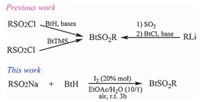
N-Sulfonylbenzotriazoles are important intermediates in organic synthesis. As the advantageous sulfonylation reagents, they can react smoothly with amines and phenols to afford the corresponding sulfonamides and sulfonates, respectively [24]. They are also easily to convert carboxylic acids into N-acylbenzotriazoles which are especially useful when the corresponding acid chlorides are difficult to obtain [25, 26]. They have a wide applicability in C-sulfonylation, and a series of α-cyanoalkyl sulfones, sulfonylheteroaromatics, α-(sulfonylalkyl) heterocycles, α-sulfonylalkyl sulfones, and esters of α-sulfonyl acids have been prepared in excellent yields, respectively [27]. They are also useful as herbicides, mutagens, insecticides, fungicides and antibacterials [28-31]. The universal methods for the preparation of N-sulfonylbenzotriazole include the reaction of sulfonyl chloride with benzotriazole (BtH) in the presence of bases [25, 32] or with 1-(trimethylsilyl) benzotriazole (BtTMS) [31] for alkyl-and arylsulfonylbenzotriazoles, and the reaction of organolithium reagents with sulfur dioxide at low temperature to obtain sulfinic acid salts, followed by the addition of N-chlorobenzotriazole (BtCl) for heteroarylsulfonylbenzotriazoles [32] (Scheme 1). However, these methods have some limitations such as hazardous starting materials, harsh reaction conditions, long reaction time and so on. Therefore, development of a general, practical and efficient method for the construction of N-sulfonylbenzotriazoles under mild conditions is still a challenge, and such a method is highly desirable. Herein, we report a convenient I2-catalyzed reaction of sodium sulfinates with benzotriazoles for the synthesis of N-sulfonylbenzotriazoles at room temperature (Scheme 1), which resulting in the target products in good yields under mild conditions.
|
Download:
|
| Scheme. 1. Methods for the synthesis of N-sulfonylbenzotriazoles. | |
2. Experimental
A typical procedure for the catalytic N-sulfonylation of benzotriazoles using I2 as catalyst includes: in EtOAc-H2O (10:1) mixture solvent (2 mL), benzotriazole 1a (0.3 mmol), sodium benzenesulfinate 2a (0.9 mmol), I2 (0.06 mmol) were added successively. The suspension mixture was vigorously stirred at room temperature for 3 h. Upon completion, the reaction was quenched by addition of sat. aq. Na2S2O3 (2 mL), basified with sat. aq. Na2CO3 (8 mL) and H2O (5 mL). The mixture was extracted with CH2Cl2 (3 × 5 mL) and the combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was then purified by TLC technique (petroleum ether/ethyl acetate=3:1, v/v) to furnish 1-phenylsulfonylbenzotriazole 3a [28] in 97% yield. Compound 3a: Pale yellow solid; mp 122-123 ℃; 1H NMR (500 MHz, CDCl3): δ 8.15-8.11 (m, 3H), 8.09 (d, 1H, J=8.4 Hz), 7.70-7.64 (m, 2H), 7.57-7.53 (m, 2H), 7.52-7.47 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 145.4, 137.0, 135.2, 131.6, 130.3, 129.6, 127.8, 125.9, 120.5, 111.9; IR (film, cm-1): ν 3094, 3069, 1586, 1480, 1386, 1194, 955, 726, 589; MS (EI, m/z, %): 260 (M + 1, 100).
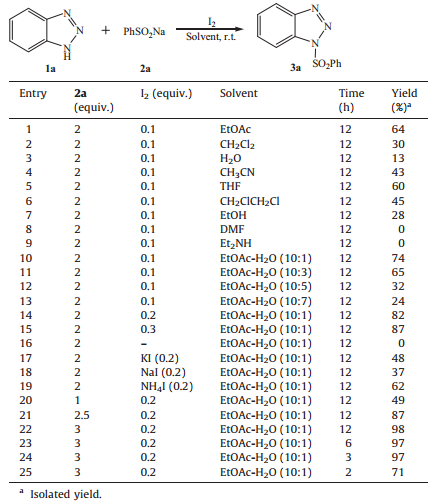
3. Results and discussionInitially, we utilized benzotriazole (1a) and sodium benzenesulfinate (2a) as model substrates to investigate the reaction for the direct preparation of 1-phenylsulfonylbenzotriazole (3a) using I2 as catalyst. It was found that simple stirring of a mixture of 0.1 equiv. of I2 with 1.0 equiv. of 1a and 2.0 equiv. of 2a in ethyl acetate (EtOAc) for 12 h at room temperature in air, the expected product 3a was obtained in 64% yield (Table 1, entry 1). Next, the procedure for the preparation of 3a catalyzed by I2 was optimized at room temperature. The reaction was first evaluated in several solvents, providing the desired product in yields ranging from 13% to 64%, in which EtOAc had the best effect (entries 1-7); while using DMF or Et2NH as solvent, 3a was not detected (entries 8 and 9). Due to the bad solubility of 2a in EtOAc, water was added in EtOAc to improve the reaction. When the volume ratio of EtOAc to H2O was 10:1, the reaction yield increased to 74%; while more H2O was added, the yields decreased (entries 10-13). Therefore, the mixed solvent EtOAc-H2O (10:1) was selected as the suitable solvent for the reaction. In the mixture solvent, the amount of catalyst I2 was evaluated and 0.2 equiv. proving the best choice; however, in the absence of it, no product was detected (entries 10, 14-16). Similar to I2, other iodine-containing catalysts such as KI, NaI and NH4I can promote the reaction, giving moderate or low yields (entries 17-19). Finally, the quantity of sodium benzenesulfinate was optimized, and 3.0 equiv. of it was the most suitable (entries 14, 20-22). Using the optimized conditions, the reaction processed smoothly and completed in 3 h (entries 22-25).
|
|
Table 1 Optimization of the N-sulfonylation of benzotriazoles using I2 as catalyst. |
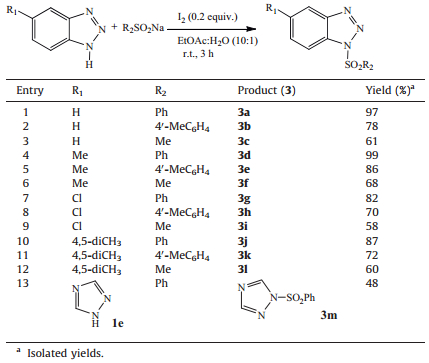
Based on the extensive screening process, we arrived at the optimal reaction conditions. Next, the catalytic reaction of 1.0 equiv. of benzotriazoles (1) with 3.0 equiv. of sodium sulfinates (2) and 0.2 equiv. of I2 in EtOAc-H2O (10:1) at room temperature for 3 h was investigated, as a consequence a series of corresponding N-sulfonylbenzotriazoles (3) were obtained. The results are summarized in Table 2.
|
|
Table 2 Preparation of N-sulfonylbenzotriazoles 3. |
As shown in Table 2, the I2-catalyzed reaction was compatible with the studied benzotriazoles 1, affording the corresponding sulfonylbenzotriazoles 3 in moderate to excellent yields (Table 2, entries 1-12). It is obvious that sodium benzenesulfinate 2a had the best effect in the reaction compared with other two sodium sulfinates, and sodium methylsulfinate 2c usually resulted in moderate yields. When 5-methylbenzotriazole 1b and 5-chlorobenzotriazole 1c were used in the reaction, these monosubstituted benzotriazoles typically gave 5-substituted and 6-substituted mixture products which were difficult to be isolated, and by 1H NMR analysis the ratios of 5-substituted products to 6-substituted products are ranging from 1:1 to 2:3 (entries 4-9). Under the same reaction conditions, 1, 2, 4-triazole 1e was also treated with 2a, but the product 3m was obtained with a somewhat low yield 48% (entry 13). To explore the efficiency and generally of our methodology, as the representative of one nitrogen atom heterocycles, indole resulted in the 2-sulfonylated product, not the desired N-sulfonylatedproduct [17].Underthe same reaction conditions, heterocycles with two nitrogen atoms and four nitrogen atoms were also checked; however, the desired N-sulfonylated products were not obtained. Therefore, the new and convenient N-sulfonylation is suitable for triazoles.
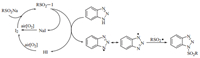
A proposed catalytic mechanism for the N-sulfonylation of benzotriazoles is shown in Scheme 2. Initially, sodium sulfinate reacts with I2 to form the active sulfonyl iodide, which is easily subjected to homolysis, giving sulfonyl and iodine radicals due to its instability [17, 33, 34]. Then, the iodine radical or sulfonyl radical attacks the hydrogen of benzotriazole to produce the benzotriazole radical, which finally reacts with sulfonyl radical to afford the N-sulfonylbenzotriazole. Meanwhile, the molecular iodine can be re-generated via the oxidation of NaI or HI by oxygen in air.
|
Download:
|
| Scheme. 2. Proposed catalytic mechanism for the N-sulfonylation of benzotriazoles. | |
To identify the mechanism, several control experiments have been made. It was found that the I2-catalyzed reaction was faster in air than in N2 atmosphere and gave higher yield, meaning oxygen in air can improve the reaction. When I2 was replaced by iodides, such as KI, NaI and NH4I, the reaction also processed (Table 1, entries 17-19); if without oxygen, the reaction was difficult to carry out, which supports above finding. That 5-methylbenzotriazole 1b and 5-chlorobenzotriazole 1c resulted in the mixture products, indicating the benzotriazole radical was formed during the reaction. As a radical scavenger, 2, 2, 6, 6-tetramethylpiperidine 1-oxyl (TEMPO) was added in the reaction and no any sulfonylbenzotriazole was detected, demonstrating that a radical mechanism is involved. We also found that simple stirring a mixture of I2 with sodium sulfinate, the product sulfonyl iodide was easily formed, after adding benzotriazole, 3a was produced quickly; however, only stirring a mixture of I2 and benzotriazole for a long time, no reaction proceeded, which indicates that sulfonyl iodide is the key intermediate in the reaction.
4. ConclusionIn summary, we have developed a new and convenient catalytic procedure for the preparation of sulfonylbenzotriazoles from benzotriazoles and sodium sulfinates in the presence of catalytic amount of I2 at room temperature in air. This sulfonylation of benzotriazoles has some advantages such as mild reaction conditions, simple procedure and giving good yields. Furthermore, the catalytic reaction will extend the application scope of molecular iodine in organic synthesis.
| [1] | Finkbeiner P., Nachtsheim B.J.. Iodine in modern oxidation catalysis. Synthesis 45 (2013) 979–999. DOI:10.1055/s-00000084 |
| [2] | Zhao J., Li P., Xia C., et al. Direct N-acylation of azoles via a metal-free catalyzed oxidative cross-coupling strategy. Chem. Commun. 50 (2014) 4751–4754. DOI:10.1039/c4cc01587h |
| [3] | Guo S., Yu J.T., Dai Q., et al. The Bu4NI-catalyzed alfa-acyloxylation of ketones with benzylic alcohols. Chem. Commun. 50 (2014) 6240–6242. DOI:10.1039/c4cc01652a |
| [4] | Wu X.F., Gong J.L., Qi X.. A powerful combination:recent achievements on using TBAI and TBHP as oxidation system. Org. Biomol. Chem. 12 (2014) 5807–5817. DOI:10.1039/C4OB00276H |
| [5] | Jia Z., Nagano T., Li X., et al. Iodide-ion-catalyzed carbon-carbon bond-forming cross-dehydrogenative coupling for the synthesis of indole derivatives. Eur. J. Org. Chem. (2013) 858–861. |
| [6] | Zeng L.Y., Yi W.B., Cai C.. Three-component domino synthesis of 2-arylquinazoline-4-amines in one pot by activating an sp3 C-H bond in a nonmetal catalytic oxidation system. Eur. J. Org. Chem. (2012) 559–566. |
| [7] | Froehr T., Sindlinger C.P., Kloeckner U., et al. A metal-free amination of benzoxazoles-the first example of an iodide-catalyzed oxidative amination of heteroarenes. Org. Lett. 13 (2011) 3754–3757. DOI:10.1021/ol201439t |
| [8] | Lamani M., Prabhu K.R.. Iodine-catalyzed amination of benzoxazoles:a metal-free route to 2-aminobenzoxazoles under mild conditions. J. Org. Chem. 76 (2011) 7938–7944. DOI:10.1021/jo201402a |
| [10] | Reddy K.R., Maheswari C.U., Venkateshwar M., et al. Oxidative amidation of aldehydes and alcohols with primary amines catalyzed by KI-TBHP. Eur. J. Org. Chem. (2008) 3619–3622. |
| [11] | Liotta D.. New organoselenium methodology. Acc. Chem. Res. 17 (1984) 28–34. DOI:10.1021/ar00097a005 |
| [12] | Tang S., Wu Y., Liao W., et al. Revealing the metal-like behavior of iodine:an iodide-catalysed radical oxidative alkenylation. Chem. Commun. 50 (2014) 4496–4499. DOI:10.1039/c4cc00644e |
| [13] | Lin Y.M., Lu G.P., Cai C., et al. Odorless, one-pot regio-and stereoselective iodothiolation of alkynes with sodium arenesulfinates under metal-free conditions in water. Org. Lett. 17 (2015) 3310–3313. DOI:10.1021/acs.orglett.5b01488 |
| [14] | Kariya A., Yamaguchi T., Nobuta T., et al. Molecular-iodine-catalyzed aerobic oxidative synthesis of β-hydroxy sulfones from alkenes. RSC Adv. 4 (2014) 13191–13194. DOI:10.1039/c3ra47863g |
| [15] | Katrun P., Hongthong S., Hlekhlai S., et al. Iodine-PPh3-mediated C3-sulfenylation of indoles with sodium sulfinates. RSC Adv. 4 (2014) 18933–18938. DOI:10.1039/c4ra02607a |
| [16] | Gao W.C., Zhao J.J., Hu F., et al. I2-mediated sulfonylation and Na2SO3-mediated deacylation:a general protocol for the synthesis of β-keto sulfones and bdicarbonyl sulfones. RSC Adv. 5 (2015) 25222–25228. DOI:10.1039/C5RA03826J |
| [17] | Katrun P., Mueangkaew C., Pohmakotr M., et al. Regioselective C2 sulfonylation of indoles mediated by molecular iodine. J. Org. Chem. 79 (2014) 1778–1785. DOI:10.1021/jo402831k |
| [18] | Xiao F.H., Chen H., Xie H., et al. Iodine-catalyzed regioselective 2-sulfonylation of indoles with sodium sulfinates. Org. Lett. 16 (2014) 50–53. DOI:10.1021/ol402987u |
| [19] | Xiao F.H., Xie H., W. S., et al. Iodine-catalyzed regioselective sulfenylation of indoles with sodium sulfinates. Adv. Synth. Catal. 356 (2014) 364–368. DOI:10.1002/adsc.201300773 |
| [20] | Yang K., Ke M., Lin Y.G., et al. Sulfonamide formation from sodium sulfinates and amines or ammonia under metal-free conditions at ambient temperature. Green Chem. 17 (2015) 1395–1399. DOI:10.1039/C4GC02236J |
| [21] | Pan X.J., Gao J., Liu J., et al. Synthesis of sulfonamides via I2-mediated reaction of sodium sulfinates with amines in an aqueous medium at room temperature. Green Chem. 17 (2015) 1400–1403. DOI:10.1039/C4GC02115K |
| [22] | Wei W., Liu C.L., Yang D.S., et al. Metal-free direct construction of sulfonamides via iodine-mediated coupling reaction of sodium sulfinates and amines at room temperature. Adv. Synth. Catal. 357 (2015) 987–992. DOI:10.1002/adsc.v357.5 |
| [23] | Chonchanok B., Danupat B., Sirilata Y.. Iodine-catalyzed oxidative amination of sodium sulfinates:a convenient approach to the synthesis of sulfonamides under mild conditions. Eur. J. Org. Chem. (2015) 1575–1582. |
| [24] | Katritzky A.R., Zhang G., Wu J.. 1-Phenylsulfonylbenzotriazole:a novel and convenient reagent for the preparation of benzenesulfonamides and aryl benzenesulfonates. Synth. Commun. 24 (1999) 205–216. |
| [25] | Katritzky A.R., He H.Y., Suzuki K.. N-Acylbenzotriazoles:neutral acylating reagents for the preparation of primary, secondary, and tertiary amides. J. Org. Chem. 65 (2000) 8210–8213. DOI:10.1021/jo000792f |
| [26] | Katritzky A.R., Shobana N., Pernak J., et al. Sulfonyl derivatives of benzotriazole:Part 1. A novel approach to the activation of carboxylic acids. Tetrahedron 48 (1992) 7817–7822. DOI:10.1016/S0040-4020(01)80459-2 |
| [27] | Katritzky A.R., Abdel-Fattah A.A.A., Vakulenko A.V., et al. N-Sulfonylbenzotriazoles as advantageous reagents for C-sulfonylation. J. Org. Chem. 70 (2005) 9191–9197. DOI:10.1021/jo051157i |
| [28] | Hergert L.Y., Nieto M.J., Becerra M.C., et al. Synthesis of N-benzenesulfonylbenzotriazole derivatives, and evaluation of their antimicrobial activity. Lett. Drug Des. Discov. 5 (2008) 313–318. DOI:10.2174/157018008784912108 |
| [29] | Soundararajan R., R. T.. Balasubramanian, Novel synthesis of potential biocides. Chem. Ind. (Lond.) 3 (1985) 92. |
| [30] | Neikova N., Simov D., Karparov A., et al. 1-(Benzenesulfonyl)benzotriazoles and intermediate products with antiviral activity, Farmatsiya (Sofia. Bulgaria) 31 (1981) 48–53. |
| [31] | Purygin P.P., Ivanov I.P., Laletina Z.P., et al. Synthesis and biological activity of N, N'-sulfuryldibenzotriazole. Khim. Farm. Zh. 17 (1983) 792–793. |
| [32] | Katritzky A.R., Rodriguez-Garcia V., Nair S.K.. A general and efficient synthesis of sulfonylbenzotriazoles from N-chlorobenzotriazole and sulfinic acid salts. J. Org. Chem. 69 (2004) 1849–1852. DOI:10.1021/jo035515y |
| [33] | Truce W.E., Wolf G.C.. Adducts ofsulfonyl iodides with acetylenes. J. Org. Chem. 36 (1971) 1727–1732. DOI:10.1021/jo00812a001 |
| [34] | Correa C.M.M.D.S., Waters W.A.. Reactions of the free toluene-p-sulphonyl radical. Part I. Diagnostic reactions of free radicals. J. Chem. Soc. C (1968) 1874–1879. |
 2016, Vol. 27
2016, Vol. 27