b Organometallic and Organometalloid Chemistry Department, National Research Centre, Dokki 12622, Egypt
Since their initial discovery, the halogen azides (IN3, ClN3, BrN3, and FN3) have been considered challenging compounds requiring skill to control/manipulate, due to their risky chemical reactivity, volatility and potential toxicity [1]. Halogen azides are highly explosive and their stability is believed to ascend in the following order: FN3 < IN3 < BrN3 < ClN3 [2]. The 1, 2-bromoazidation of alkenes was first reported by Hassner and Boerwinkle [3] by the addition of bromine azide generated from bromine and sodium azide. Subsequently, Hassner and co-workers [4-8] made a major contribution to understanding the mechanism of halogen azide addition to olefins and its regiospecificity through two different pathways, namely a radical or an ionic process. Also, BrN3 was synthesized by many in situ systems starting from [Br2 gas/NaN3] which was modified by Hassner to [Br2-HCl-NaN3] [3-5], hydrazoic acid [NXS-HN3, X=I, Br] [9] and [Br2/NaN3 in base] [10]. However, most of these methods have significant hazards related to explosivity and toxicity. The convenience and versatility of in situ techniques were initiated by Krief in 1974 [NBS-NaN3] [11-16], [NBS-TMSN3] [17] and [metal triflates-NBS-TMSN3] [18-20].Lastly, several sources of Br+ such as TsNBr2 [21] and PhNMe3Br3 [22] have been used with trimethylsilylazide. Many of the described protocols exhibit lack of selectivity, low-yield and require toxic or exotic reagents. So, the [NBS-TCSN3] reaction system is anticipated as a dependable option for bromoazidation. Haloazidation represents an important transformation in organic synthesis in which vicinal haloazide compounds are versatile precursors of vinyl azides [23], amines [24], heterocycles [25] and particularly aziridines [26]. Therefore, the haloazidation of olefinic double bonds still remains an important and challenging task for organic chemists.
2. Experimental 2.1. Typical procedure for the synthesis of azidobromoarylidine-malononitrileIn a dry, two-neck, round bottom flask equipped with a rubber septum, a magnetic stirring bar and a condenser and surrounded by a light excluding jacket, a mixture of TCS (10 mmol), and sodium azide (10 mmol) was stirred for 10 min, then NBS (10 mmol) and benzalmalononitrile (10 mmol) in CH3CN (15 mL) were added. This mixture was allowed to stir with the exclusion of moisture at r.t. for the specified time. The mixture was then poured into icecold H2O (~100 mL) and extracted with diethyl ether (2 × 25 mL), then the extract was dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the obtained product was collected and identified after drying.
Data for representative examples are shown:
2-Azido-1-bromo-1, 1-dicyano-2-(4-bromophenyl)ethane (d): Mp 93-95 ℃; IR (KBr, cm-1): ν 2934, 2899 (CH), 2224 (CN), 2122 (N3). 1H NMR (300 MHz, CDCl3): δ 7.77-7.73 (d, 2H, J=7.8 Hz, ArH), 7.52-7.44 (d, 2H, J=7.8 Hz, ArH), 5.10 (s, 1H, CH); 13C NMR (75 MHz, CDCl3): d 132.92, 132.50, 131.71, 129.99 (Ar-C), 110.03 (2CN), 69.86 (CH), 29.88 (C); MS (EI 70 eV) m/z: 353 (M+); Anal. Calcd. for C10H5Br2N5 (352.89): C, 33.83; H, 1.42; Br, 45.02; N, 19.73. Found: C, 33.80; H, 1.36; Br, 45.00; N, 19.65.
2, 4-Diazido-1, 3-dibromo-1, 1-dicyano-4-phenylbutane (g): Mp. 135 ℃; IR (KBr, cm-1): ν 2934 (CH), 2250 (CN), 2118 (N3); 1H NMR (300 MHz, CDCl3): δ 7.55-7.35 (m, 5H, ArH), 5.10-5.15 (d, 2H, J=18 Hz), 5.08-5.05 (d, 2H, J=18 Hz), 4.80-4.65 (dd, 1H, J=18 Hz); 13C NMR (75 MHz, CDCl3): δ 136, 129, 128.52, 127 (Ar-C), 109.50, 108.50 (2CN), 69 (C-4), 62 (C-2), 52 (C-3), 28.50 (C-1); MS (EI 70 eV) m/z: 422 (M+); Anal. Calcd. for C12H8Br2N8 (421.92): C, 33.99; H, 1.90; Br, 37.69; N, 26.42. Found: C, 33.93; H, 1.85; Br, 37.63; N, 26.35.
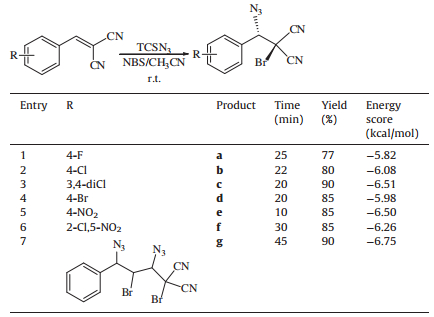
3. Results and discussion 3.1. ChemistryDuring last decade, several research groups used TCS as a catalyst in synthetic organic chemistry [27]. Also, TCS and silzic play a significant role in our research group activities [28, 29]. In conjunction with our interests, we present, in this work, the utilization of bromine azide to access 1, 2-azidobromides as an anti-Markovinkov addition product of the olefinic double bond in benzalmalononitriles. The reaction occurs by mixing TCS/NaN3 at a molar ratio 1:1 for 10 min, then adding 1 mol of NBS and 1 mol of benzalmalononitrile in acetonitrile as a solvent and stirring for the appropriate time at r.t. (Scheme 1).
|
Download:
|
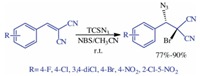
| Scheme. 1. Synthesis of 1, 2-azidobromides from arylidene malononitrile. | |
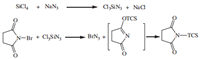
Although BrN3 generated in situ is soluble in organic solvents [2], many solvents (CH2Cl2, CH3NO2, CH2Cl2 + CH3NO2, n-hexane and acetonitrile) were examined and it was determined that acetonitrile has higher solubility as a starting material, less reaction time, high reaction yield and easy work up and also that BrN3 is sensitivity to light and so the reaction is run with a jacketed flask to exclude light. The suggested mechanism for the formation of bromine azide is explained in Scheme 2 [30].
|
Download:
|
| Scheme. 2. In situ generation of BrN3. | |
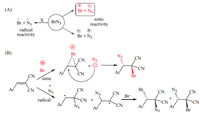
The proposed reaction mechanism between azidobromides with benzalmalononitrile is explained by two pathways [31]: Pathway A shows the BrN3 fragmentation by a radical or an ionic substituent as explained by Hassener and coworker. In the presented reaction, the radical fragmentation was excluded because the reaction did not give mixed products and the reaction is done in dark jacket; Pathway B shows the anti-addition step of BrN3 to benzalmalononitrile (Scheme 3).
|
Download:
|
| Scheme. 3. (A) Fragmentation pathways for BrN3. (B) Formation of 1, 2-azidobromides. | |
In order to support the validity and regioselectivity of the reaction reagent, the data in Table 1 summarizes a preliminary survey of the vicinal azidobromination of benzalmalononitrile. All of the substrates (entries 1-6) underwent anti-Markovnikov addition type reaction where the electron withdrawing power of the two CN groups shift the electron density to the a-position and from this the radical mechanism becomes excluded. Also, the reaction was done with cinnamalmalononitrile (entry 7) using two equivalents of reagents under the same conditions and after 45 min, the 2, 4-diazido-1, 3-dibromo-1, 1-dicyano-4-phenyl (g) was obtained in excellent yield proving the efficacy and regioselectivity of this reagent.
|
|
Table 1 Bromoazidation for various arylidenes malononitrile. |
The structures of the resulting vicinal azidobrominations were elucidated by spectroscopic methods. The IR spectrum showed peaks at ν=2250-2215, 2126-2118 cm-1 corresponding to CN and N3, respectively. The 1H NMR spectrum of the synthesized products revealed a doublet signal for (1H) in CH at δ 5.07-5.85 and at δ 8.75-7.29 refers to ArCH ppm. From the results presented in Table 1, the reaction times were short (10-30 min) and the yields were high (77%-90%) for all products, especially in the presence of strong electron withdrawing group like nitro group. In the case of fluorine substitution, the mesomeric effect of the 2p orbital of the aromatic ring decreases the withdrawing power electronegativity of fluorine atom rather than 3p orbital of chlorine and 4p orbital of bromine, and also the steric hindrance of Cl and NO2 groups in the ortho position of the aromatic ring play the main role in increasing the reaction time in the case of compound f.
3.2. Molecular docking methodologyThiamine pyrophosphate (TPP) riboswitches regulate essential genes in bacteria by changing conformation upon binding intracellular TPP. Riboswitches are promising antimicrobial targets because of their highly specific small molecule recognition prevalent in bacteria, and the association with genes essential for pathogen survival or virulence. And it was noticed that most ligands used against protein targets only recently has this approach been applied to RNA, leading to the discovery of several small molecules that bind the Escherichia coli thiM TPP riboswitch. Thus, even though further substantial development is necessary, fragments have the potential to be elaborated in ligands specific for TPP riboswitches. The standard docking protocol using MOE 2015.10 software was used [32] to study the interaction between the prepared ligand molecules (a-g) and TPP riboswitches. From the validation based on comparing the calculated binding energy scores (kcal/mol) of the prepared ligands (Table 1) and energy score (-6.43 kcal/mol) of the reference ligand [33], there are three ligands (c, e and g) which give less energy scores related to the strong interaction with TPP riboswitch binding sites by forming eight hydrogen bonds.
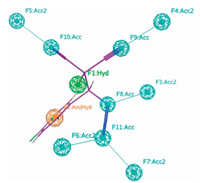
The selection of vicinal bromoazide scaffold analogs (a-g) to build a pharmacophore for potential Aurora kinase A inhibitors was based on their high potency. The superposition of model example e was generated a pharmacophore (Fig. 1).
|
Download:
|
| Figure 1. TPP pharmacophore model with compound e (H atoms in gray, C atoms in green, O atoms in red, Br atom in deep red colors and N atoms in blue colors). F1: Hyd stands for hydrophobic center; F2: Aro for aromatic center; F(3–7): Acc2 for Hbond acceptor projection and F(8–11): Acc=H-bond acceptor. | |
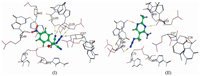
The active site analysis of the TPP riboswitches RNA was performed from MOE database similar nucleotide residues receptor as C57, U20, C73, G19, G42, G72, A43, G40, U39 and C58. The interaction between ligand compound e and TPP pocket residue was done by eight hydrogen bonds; the Br atom attached C73 by distance 3.76 Å and energy -1.2 kcal/mol, the N atom in azide attached with C57, G19, G19 by distance (3.51, 3.08 and 3.50 Å, respectively) and energy (-2.4, -5.7 and -2.0 kcal/mol, respectively), the nitrogen atom in cyano group attached U20 and G19 by distance (3.38 and 2.94 Å, respectively) and energy (-1.2 and -2.1 kcal/mol, respectively) and the oxygen atom in nitro groups attached G40 by distance 3.02 Å and energy -2.4 kcal/mol. Also, the interaction between TPP and reference ligand was explained in 3D-structure by forming four hydrogen bonds; nitrogen in azide group with U39 and C73 (3.29 and 4.05 Å respectively), nitrogen in pyrimidine ring with G19 and G40 (3.30 and 3.22 Å respectively) (Fig. 2).
|
Download:
|
| Figure 2. Docked 3D-structure for hydrogen bond (yellow color) interaction between TPP and compound e (Ⅰ) and the reference ligand (]Ⅱ). | |
4. Conclusion
Vicinal bromoazide was synthesized from arylidene malononitrile. Thr reaction was started by the generation of BrN3 in situ by the interaction between sodium azide, N-bromosuccinimide and TCS in acetonitrile as a solvent at ambient temperature. This catalytic process represents a highly regioselective method for the synthesis of 1, 2-bromoazides. The reaction products were obtained in excellent yield and characterized by IR, MS, 1H NMR, 13C NMR, and elemental analysis. The molecular docking for the synthesized molecules was performed to prove that the synthesized molecules gave strong interaction with TPP riboswitches compared with 5-(azidomethyl)-2-methylpyrimidin-4-amine as the reference molecule.
| [1] | Bauer S.H.. An electron diffraction investigation of the structure of difluorodiazine. J. Am. Chem. Soc. 69 (1947) 3104–3108. DOI:10.1021/ja01204a053 |
| [2] | Dehnicke K.. Reactions of halogen azides. Angew. Chem. Int. Ed. Engl. 6 (1967) 240–246. DOI:10.1002/(ISSN)1521-3773 |
| [3] |
(a) A. Hassner, F. Boerwinkle, Ionic and free-radical addition of bromine azide to olefins, J. Am. Chem. Soc. 90(1968) 216-218; (b) F. Boerwinkle, A. Hassner, Solvent participation in additions to olefin, Tetrahedron Lett. 9(1968) 39213924; (c) A. Hassner, F. Boerwinkle, Free radical vs ionic additions of halogen azides to olefins, Tetrahedron Lett. 10(1969) 3309-3319. |
| [4] | Hassner A., Teeter J.S.. Stereochemistry. LIV. Phenyl migration in pseudo halogen additions to 3, 3, 3-triphenylpropene. J. Org. Chem. 35 (1970) 3397–3401. DOI:10.1021/jo00835a046 |
| [5] | Hassner A.. Regiospecific and stereospecific introduction of azide functions into organic molecules. Acc. Chem. Res. 4 (1971) 9–16. DOI:10.1021/ar50037a002 |
| [6] | L'Abbe G., Hassner A.. Synthesis of a-azidovinyl ketones from the iodine azide adducts of a, b-unsaturated ketones. J. Org. Chem. 36 (1971) 258–260. DOI:10.1021/jo00801a004 |
| [7] | L'Abbé G., Hassner A.. Neue methoden zur darstellung von vinylaziden. Angew. Chem. Int. Ed. Engl. 10 (1971) 98–104. DOI:10.1002/anie.197100981 |
| [8] | Hassner A., Keogh J.. Addition of halogen azides to non-cyclic conjugated dienes. Tetrahedron Lett. 16 (1975) 1575–1578. DOI:10.1016/S0040-4039(00)72201-5 |
| [9] | Ponsold K., Wunderwald M.. Gekoppelte additionsreaktionen an 14, 15-ungesättigten androstanen einfluß des positiven halogens auf die regioselektivität. J. Prakt. Chem. (Leipzig) 325 (1983) 123–132. DOI:10.1002/prac.19833250115 |
| [10] | Nagorski R.W., Brown R.S.. Electrophilic addition of bromine to olefins in the presence of nucleophilic trapping anions. Implications for the lifetimes of bromonium ion intermediates produced from electrophilic bromination of olefins in methanol. J. Am. Chem. Soc. 114 (1992) 7773–7779. DOI:10.1021/ja00046a024 |
| [11] | Van Ende D., Krief A.. A new reagent for stereospecific synthesis of aziridines from olefins. Angew. Chem. Int. Ed. Engl. 13 (1974) 279–280. |
| [12] | Denis J.N., Krief A.. New synthetic route to 9, 10-imino-phenanthrene. Tetrahedron 35 (1979) 2901–2903. DOI:10.1016/S0040-4020(01)99506-7 |
| [13] | Chiu I.C., Kohn H.. Syntheses and reactivity of trans-6-azabicyclo[3.1.0] hexan-2-ol derivatives and indanol[1, 2-b]aziridine. Structural analogs of mitomycin C. J. Org. Chem. 48 (1983) 2857–2866. DOI:10.1021/jo00165a015 |
| [14] | Nguy N.M., Chiu I.C., Kohn H.. Synthesis and reactivity of 6-and 7-methoxyindano[1, 2-b]aziridines. J. Org. Chem. 52 (1987) 1649–1655. DOI:10.1021/jo00385a001 |
| [15] | Lange W., Tueckmantel W.. Synthesis and reactions of oligomethylene-clamped 1 H-azepines and benzene imines. Their valence tautomeric equilibrium and nitrogen stereochemistry. Chem. Ber. 122 (1989) 1765–1976. DOI:10.1002/(ISSN)1099-0682 |
| [16] | Devalankar D.A., Sudalai A.. A concise synthesis of (+)-deoxoprosophylline via Co(Ⅲ)(salen)-catalyzed two stereocentered HKR of racemic azido epoxides. Tetrahedron Lett. 53 (2012) 3213–3215. DOI:10.1016/j.tetlet.2012.04.067 |
| [17] | Saikia I., Phukan P.C.. Catalyst-free vicinal bromoazidation of olefins using TMSN3 and NBS. C.R. Chim. 15 (2012) 688–692. DOI:10.1016/j.crci.2012.05.001 |
| [18] | Hajra S., Sinha D., Bhowmick M.. Metal triflate catalyzed highly regio-and stereoselective 1, 2-bromoazidation of alkenes using NBS and TMSN3 as the bromine and azide sources. Tetrahedron Lett. 47 (2006) 7017–7019. DOI:10.1016/j.tetlet.2006.07.110 |
| [19] | Hajra S., Sinha D., Bhowmick M.. Metal triflate catalyzed reactions of alkenes, NBS, nitriles, and TMSN3:synthesis of 1, 5-disubstituted tetrazoles. J. Org. Chem. 72 (2007) 1852–1855. DOI:10.1021/jo062432j |
| [20] | Hajra S., Bhowmick M., Sinha D.. Highly regio-and stereoselective asymmetric bromoazidation of chiral a, b-unsaturated carboxylic acid derivatives:scope and limitations. J. Org. Chem. 71 (2006) 9237–9240. DOI:10.1021/jo061593k |
| [21] | Saikia I., Phukan P.. Facile generation of vicinal bromoazides from olefins using TMSN3 and TsNBr2 without any catalyst. Tetrahedron Lett. 50 (2009) 5083–5087. DOI:10.1016/j.tetlet.2009.06.059 |
| [22] | Kumar A., Rao M.S., Mehta V.. A simple and efficient bromoazidation of alkenes using PTT and TMSN3 in ionic liquid. Indian, J. Chem., Sect. B:Org. Chem. Incl. Med. Chem. 50B (2011) 1123–1127. |
| [23] | Hassner A., Fowler F.W.. General synthesis of vinyl azides from olefins. Stereochemistry of elimination from b-iodo azides. J. Org. Chem. 33 (1968) 2686–2691. DOI:10.1021/jo01271a016 |
| [24] | Wasserman H.H., Brunner R.K., Buynak J.D., et al. Total synthesis of (±)-Omethylorantine. J. Am. Chem. Soc. 107 (1985) 519–521. DOI:10.1021/ja00288a050 |
| [25] |
(a) S. Ranganathan, D. Ranganathan, A.K. Mehrotra, The "Hassner-Ritter" reaction in iodine azide additions to pinenes with solvent participation, Tetrahedron Lett. 14(1973) 2265-2266; (b) S.N. Moorthy, D. Devaprabakara, Addition of iodine azide to C-9 and C-10 medium-ring dienes, Tetrahedron Lett. 16(1975) 257-260. |
| [26] |
(a) D. Van Ende, A. Krief, A highly potent reagent for regioselective and stereospecific synthesis of polyenes bearing terminal aziridine groups, Angew. Chem. Int. Ed. Engl. 13(1974) 279-280; (b) J.N. Denis, A. Krief, New synthetic route to 9, 10-imino-phenanthrene 1, Tetrahedron 35(1979) 2901-2903. |
| [27] | Rossi S., Benaglia M., Genoni A.. Organic reactions mediated by tetrachlorosilane. Tetrahedron 70 (2014) 2065–2080. DOI:10.1016/j.tet.2014.01.055 |
| [28] |
(a) H.A. Soliman, T.K. Khtab, Efficient heterogeneous catalytic one-pot, three component synthesis of γ-hydroxy-β-ketoamide, Egypt J. Chem. 57(2014) 129-142; (b) T.K. Khatab, K.A.M. El-Bayouki, W.M. Basyouni, A new and facile tetrachlorosilane-promoted one-pot condensation for the synthesis of a novel series of tetracyclic 1, 5-thiazepines, Tetrahedron Lett. 55(2014) 6039-6041; (c) H.A. Soliman, T.A. Salama, Silicon-mediated highly efficient synthesis of 1, 8-dioxo-octahydroxanthenes and their transformation to novel functionalized pyrano-tetrazolo[1, 5-a] azepine derivatives, Chin. Chem. Lett. 24(2013) 404-406; (d) T.K. Khatab, K.A.M. El-Bayouki, W.M. Basyouni, An efficient synthesis of b-acylureas via a three-component, one-potsynthesis using TCS/ZnCl2, Tetrahedron Lett. 52(2011) 1448-1451; (e) D.S. Badawy, E. Abdel-Galil, E.M. Kandeel, W.M. Basyouni, T.K. Khatab, Tetrachlorosilane-zinc chloride as a new potent binary reagent for one-pot, threecomponent synthesis of Mannich-type products, Phosphorus Sulfur Silicon Relat. Elem. 184(2009) 2799-2812; (f) S.S. Elmorsy, D.S. Badawy, T.K. Khatab, Chemoselective bromination in a twostep substitution under the influence of tetrachlorosilane and N-bromosuccinimide, Phosphorus Sulfur Silicon Relat. Elem. 181(2006) 2005-2012. |
| [29] |
(a) H.A. Soliman, A.Y. Mubarak, S.S. Elmorsy, An efficient synthesis of bis(indolyl) methanes and N, N'-alkylidene 4 bisamides by Silzic under solvent free conditions, Chin. Chem. Lett. 27(2016) 353-356; (b) H.A. Soliman, A.Y. Mubarak, A. El-Mekabati, H.M. Awad, S.S. Elmorsy, Ecofriendly synthesis of amidochloroalkyl naphthols, cyclization of the product to oxazepinones for biological evaluation, Monatsh Chem. 147(2016) 809-816; (c) H.A. Soliman, A.Y. Mubarak, A. El-Mekabati, S.S. Elmorsy, SiO2/ZnCl2-catalyzed heterocyclic synthesis:green, rapid and efficient one-pot synthesis of 14-H-dibenzo[a, j]xanthenes, 1, 8-dioxo-octahydroxanthenes and 1, 8-dioxo-decahydroacridines under solvent-free conditions, Chem. Sci. Trans. 3(2014) 819-825. |
| [30] | Olah G.A., Wang Q., Li X.Y., Prakash G.K.S.. Azidobromination of alkenes with azidotrimethylsilane/n-bromosuccinimide 1. Synlett (1990) 487–489. |
| [31] |
(a) R.A. Valiulin, S. Mamidyala, M.G. Finn, Taming chlorine azide:access to 1, 2-azidochlorides from alkenes, J. Org. Chem. 80(2015) 2740-2755; (b) D. Cantillo, B. Gutmann, C.O. Kappe, Safe generation and use of bromine azide under continuous flow conditions-selective 1, 2-bromoazidation of olefins, Org. Biomol. Chem. 14(2016) 853-857. |
| [32] | The Molecular Operating E, Chemical Computing Group I, Montreal, QC, 2015. |
| [33] | Warner K.D., Homan P., Weeks K.M., et al. Validating fragment-based drug discovery for biological RNAs:lead fragments bind and remodel the TPP riboswitch specifically. Chem. Biol. 21 (2014) 591–595. DOI:10.1016/j.chembiol.2014.03.007 |
 2016, Vol. 27
2016, Vol. 27