The development of general and mild protocol for disulfides synthesis has received significant attention because they are indispensable in many important synthetic chemistry [1-3], biochemistry [4] and industrial applications [5]. Various protocols have been developed for the preparation of disulfides. They can be classified as two major strategies. Firstly, a number of different methods have been devised with various sulfur-transfer agents, such as arenesulfonyl chlorides [6], CS2 [7], 1, 3-thiazolidinedione [8], potassium 5-methyl-1, 3, 4-oxadiazole-2-thiolate [9], thiourea [10], S [11], thioacetamide [12] and bunte salts[13]. While, oxidative coupling of thiols with stoichiometric oxidation or catalytic oxidation has become a routine and elegant protocol for the synthesis of disulfides in recent years, primarily because a large number of thiols are easily synthesized or commercially available. Various reagents, such as N-phenyltriazolinedione [14], Fe(NO3)3·9H2O/Fe(HSO4)3 [15], 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ) [16], tributylammonium halochromates/silica gel [17], Burgess reagent [18], ceric ammonium nitrate (CAN) [19], sulfuryl chloride [20], cetyltrimethylammonium dichromate [21], bromate [22] and N2O4/PVP [23] have been used as stoichiometric oxidants. Early studies in this field have one or more disadvantages, such as long reaction time, difficult work-up, use of toxic or costly reagents, low yield of product due to over oxidation, etc. Therefore, it is still necessary to develop mild, efficient and environmentally friendly methods to synthesize disulfides from thiols.
In this way, many chemists pay much attention to discover ecofriendly catalytic oxidation in recent years. With the development of green and sustainable chemistry, photocatalytic oxidation of thiols to disulfides appears as an interesting option and some publications have been introduced in this field [24]. Furthermore, some elegant routes with the use of H2O2 as the green oxidant have been reported [25]. Molecular oxygen is also well known to be an economical, non-toxic, and easy-handle oxidant for the oxidation of thiols into disulfides [26]. These aerobic oxidation of thiol are performed in the presence of Co-salophen [26a], Fe(BTC) [26b], Au/CeO2 [26c], iron(Ⅲ)-ethylenediaminetetraacetic acid [26d], [hmim]Br [26e], CoPcS@ASMNP [26f], amino acid [26g], Ag2O nanoparticles/silica [26h], selenium ionic liquid [26i], Na2CO3/ BMIM-BF4[26j], N2O4/charcoal [26k] and Fe(CF3CO2)3 [26l]. Among them, the protocol of N2O4/charcoal has attracted our attention though it is difficult to impregnate liquid N2O4 on charcoal. From the practical viewpoint, it is essential to investigate the introduction and application of new member of catalysts in the synthesis of disulfides from thiols. For example, alkyl nitrites, which are valuable reagents in organic synthesis [27], came to our sight. Previously, our group utilized tert-butyl nitrite (TBN) as the cocatalyst in the TEMPO or DDQ-catalyzed aerobic oxidation [28]. Herein we report that the TBN/O2 system can proceed well in the disulfides synthesis from thiols. To the best of our knowledge, this work is the first example of conversion of thiols to disulfides catalyzed by TBN with molecular oxygen as the oxidant.
2. ExperimentalAll the chemicals were purchased commercially and used without any further treatment, unless otherwise stated. Benzenethiol (1a) was synthesized according to literature [29]. GC analyses were conducted on an Agilent GC6890N system with a flame ionization detector (FID) and an FFAP or OV-17 capillary column. Conversions and selectivities were determined by area normalization. 1H NMR and 13C NMR spectra were carried out on a Bruker Avance Ⅲ (500 MHz) spectrometer. CDCl3 and DMSO-d6 were used as the solvents with tetramethylsilane (TMS) as the internal standard. GCMS was performed on Finnigan Trace GC Ultra-Finnigan Trace DSQ instrument. Low resolution mass spectra were recorded in the ESI mode on an Agilent 6210 LC/TOF mass spectrometer. Melting points were measured using Buchi melting pointing M-565.
Typical procedure for disulfides (2a): A sealed tube (90 mL) equipped with a magnetic stirring bar and an O2 balloon was charged with dichloroethane (DCE, 20 mL), thiophenol (1a, 4 mmol, 0.44 g) and TBN (0.16 mmol, 4 mol%, 19.2 μL). Then the tube was placed in an oil bath, which was preheated to 50 ℃. The mixture was stirred for 1 h until starting material was completely consumed as monitored by GC and TLC. After removing the solvent, the residue was purified by column chromatography on silica gel to give the desired diphenyl disulfide (2a, 90%, 0.394 g) as a white solid.
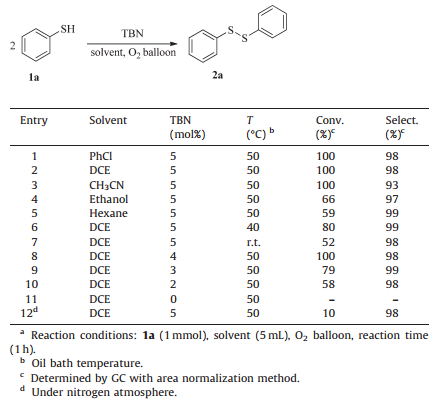
3. Results and discussionWe started to optimize the process with thiophenol (1a) as the model substrate (Table 1). In a first set of experiments (entries 1-5), the influence of different solvents was studied. Among the tested solvents, 1a could be completely oxidized into diphenyl disulfide (2a) in excellent selectivity with 5 mol% TBN under atmospheric pressure of O2 within 1 h in chlorobenzene and dichloroethane (entries 1 and 2). When acetonitrile was used as the solvent, the selectivity to 2a was decreased to 93% (entry 3). In other screened solvents, such as ethanol and n-hexane, the reaction was not successful (entries 4 and 5). The low conversion of 1ain n-hexane might be due to the poor solubility of 1a in n-hexane. Asaresult, dichloroethane(DCE)was chosen as the reaction solvent. After ward, the effect of temperature was investigated. Results indicated that decreasing temperature from 50 ℃ to room temperature, the conversion rate of 1a dropped sharply (entry 7). Perhaps TBN could not be decomposed completely at room temperature, and only partial NO was released which was the active specie in this catalytic oxidation reaction.Later on, the loads of TBN was attempted to be reduced. When the load of TBN was reduced from 5 mol% to 4 mol%, 1a could also be fully converted to 2a in 98% selectivity (entry 8). However, decreasing the load of TBN tO 3 mol%or 2 mol%, conversionof 1a was 79% and 58%, respectively (entries 9-10).Just as we have expected, there action did not occur in the absence of TBN (entry 11). In addition, under nitrogen atmosphere only 10% conversion of 1a was obtained (entry 12). On the basis of these experimental data, weconcludedthat4 mol% of TBN in DCE at 50 ℃ was suitable for oxidation of thiols to disulfides under atmospheric pressure of O2.
|
|
Table 1 Oxidative coupling of 1a into 2a.a |
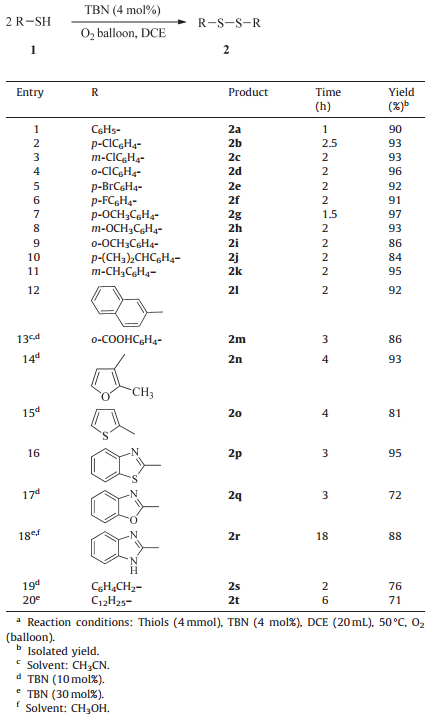
Using the optimized reaction conditions, the present method could be used to convert a wild range of commercially available thiols into their corresponding disulfides. As shown in Table 2, all thiol substrates could be smoothly converted to corresponding disulfides with excellent yields without any evidence for the formation of sulfonic acids under mild conditions in short time. All aromatic thiols were more active and provided better product yields than those of heteroaromatic thiols and aliphatic thiols. The steric effects and electronic effects in aromatic thiols did not play important roles in the isolated yield of the desired products (entries 2-12). In addition, oxidative coupling reaction using thiosalicylic acid (1m) as the substrate successfully took place under present catalytic conditions. What is more, heteroaromatic thiols (1n-1r) also could be successfully converted into desired products (2n-2r) (entries 13-18), although higher loading of TBN was needed (entries 13-15 and 17). For 2-mercaptobenzimidazole (1r), whichwas difficult to dissolve in most of solvents, the amount of TBN needed to be increased to 30 mol% to complete the reaction (entry 18). Finally, aliphatic thiols also could produce the target products in moderate yields (entries 19 and 20).
|
|
Table 2 Oxidative coupling of various thiols to disulfides catalyzed by TBN.a |
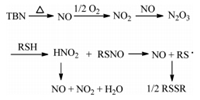
According to the literature [30], a plausible reaction mechanism for TBN-catalyzed aerobic oxidation of thiols to disulfides has been proposed (Scheme 1). TBN releases NO under 50 ℃, and then NO is easily oxidized by oxygen to form NO2. NO2 reacts with NO to form N2O3, which reacts with RSH to form unstable RSNO and HNO2. RSSR get from the hemolytic cleavage of the sulfur-nitrogen bond of the RSNO and then coupling of the resultant thiyl radicals.
|
Download:
|
| Scheme. 1. Plausible mechanism of TBN-catalyzed aerobic oxidation of thiols to disulfides. | |
4. Conclusion
In conclusion, we have successfully developed a mild and convenient catalytic approach for the oxidative coupling of thiols to the corresponding disulfides. The significant features of our protocol are metal-free, atom efficiency, using of molecular oxygen as the terminal green oxidant. Furthermore, the isolation of the products is remarkably easy.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21376224, 21206147).
| [1] | Liang G.G., M.C.Liu, Chen J.X., et al. NBS-promoted sulfenylation of sulfinateswith disulfides leading to unsymmetrical or symmetrical thiosulfonates. Chin. J. Chem. 30 (2012) 1611–1616. DOI:10.1002/cjoc.v30.7 |
| [2] |
(a) D. Huang, J. Chen, W. Dan, et al., A metal-free sulfenylation and bromosulfenylation of indoles:controllable synthesis of 3-arylthioindoles and 2-bromo-3-arylthioindoles, Adv. Synth. Catal. 354(2012) 2123-2128; (b) W. Ge, Y. Wei, Iodine-catalyzed oxidative system for 3-sulfenylation of indoles with disulfides using DMSO as oxidant under ambient conditions in dimethyl carbonate, Green Chem. 14(2012) 2066-2070. |
| [3] | Srogl J., Hyvl J., Revesz A., et al. Mechanistic insights into a copper-disulfide interaction in oxidation of imines by disulfides. Chem. Commun. (2009) 3463–3465. |
| [4] |
(a) G. Saito, J.A. Swanson, K.-D. Lee, Drug delivery strategy utilizing conjugation via reversible disulfide linkages:role and site of cellular reducing activities, Adv. Drug Deliv. Rev. 55(2003) 199-215; (b) M.C. Fournie-Zaluski, P. Coric, S. Turcaud, et al., Potent and systemically active aminopeptidase N inhibitors designed from active-site investigation, J. Med. Chem. 35(1992) 1259-1266. |
| [5] |
(a) K. Ramadas, N. Srinivasan, Sodium chlorite-yet another oxidant for thiols to disulphides, Synth. Commun. 25(1995) 227-234; (b) A. Leitao, C. Costa, A.Rodrigues, Studieson theimpregnationstep ofthe merox process, Chem. Eng. Sci. 42(1987) 2291-2299. |
| [6] |
(a) Y. Liu, Y. Zhang, Temperature-controlled selective reduction of arenesulfonyl chlorides promoted by samarium metal in DMF, Tetrahedron Lett. 44(2003) 4291-4294; (b) G.W. Kabalka, M.S. Reddy, M.L. Yao, Synthesis of diaryl disulfides via the reductive coupling of arylsulfonyl chlorides, Tetrahedron Lett. 50(2009) 7340-7342. |
| [7] |
(a) F. Barba, F. Ranz, B. Batanero, Electrochemical transformation of diazonium salts into diaryl disulfides, Tetrahedron Lett. 50(2009) 6798-6799; (b) H. Firouzabadi, N. Iranpoor, A. Samadi, One-pot synthesis ofaryl alkyl thioethers and diaryl disulfides using carbon disulfide as a sulfur surrogate in the presence of diethylamine catalyzed by copper(I) iodide in polyethylene glycol (PEG200), Tetrahedron Lett. 55(2014) 1212-1217. |
| [8] | Sun J., Xia E.Y., Wu Q., et al. Synthesis of ammonium S-S bond linked dipyridinedionates via four-component reactions of cyanoacetamide, aldehyde, amine and 1, 3-thiazolidinedione. Tetrahedron 66 (2010) 7794–7798. DOI:10.1016/j.tet.2010.07.051 |
| [9] | M.Soleiman-Beigi, F.Mohammadi. A novelcopper-catalyzed, one-pot synthesisof symmetric organic disulfides from alkyl and aryl halides:potassium 5-methyl-1, 3, 4-oxadiazole-2-thiolate as a novel sulfur transfer reagent. Tetrahedron Lett. 53 (2012) 7028–7030. DOI:10.1016/j.tetlet.2012.10.016 |
| [10] | Khalili D.. Graphene oxide-assisted one-pot and odorless synthesis of symmetrical disulfides using primary and secondary alkyl halides (Tosylates) and thiourea as sulfur source reagent, Phosphorus. Sulfur Silicon Realt. Elem. 190 (2015) 1727–1734. DOI:10.1080/10426507.2014.999069 |
| [11] |
(a) J.T. Yu, H. Guo, Y. Yi, et al., The chan-lam reaction of chalcogen elements leading to aryl chalcogenides, Adv. Synth. Catal. 356(2014) 749-752; (b) Z. Li, F. Ke, H. Deng, et al., Synthesis of disulfides and diselenides by coppercatalyzed coupling reactions in water, Org. Biomol. Chem. 11(2013) 2943-2946. |
| [12] | Soleiman-Beigi M., Hemmati M.. An efficient, one-pot and CuCl-catalyzed route to the synthesis of symmetric organic disulfides via domino reactions of thioacetamide and aryl (alkyl) halides. Appl. Organometal. Chem. 27 (2013) 734–736. DOI:10.1002/aoc.v27.12 |
| [13] | Mokhtari B., Kiasat A.R., Monjezi J.. Imidazole promoted highly efficient largescale thiol-free synthesis of symmetrical disulfides in aqueous media, Phosphorus. Sulfur Silicon Realt. Elem. 190 (2015) 1573–1579. DOI:10.1080/10426507.2014.1003643 |
| [14] | Christoforou A., Nicolaou G., Elemes Y.. N-Phenyltriazolinedione as an efficient, selective, and reusable reagent for the oxidation of thiols to disulfides. Tetrahedron Lett. 47 (2006) 9211–9213. DOI:10.1016/j.tetlet.2006.10.134 |
| [15] | Shirini F., Zolfigol M.A., Abri A.R.. Fe(NO3)3·H2O/Fe(HSO4)3:an efficient reagent system for the oxidation of alcohols, thiols and sulfides in the absence of solvent. Chin. Chem. Lett. 19 (2008) 51–54. DOI:10.1016/j.cclet.2007.11.023 |
| [16] | Vandavasi J.K., Hu W.P., Chen C.Y., et al. Efficient synthesis of unsymmetrical disulfides. Tetrahedron 67 (2011) 8895–8901. DOI:10.1016/j.tet.2011.09.071 |
| [17] | Kazem M.M., Shahriare G., Soroush Z.. Tributylammonium halochromates/silica gel:simple reagents for oxidative coupling of thiols to symmetrical disulfides. Chin. J. Chem. 28 (2010) 2199–2203. DOI:10.1002/cjoc.201090363 |
| [18] | Banfield S.C., Omori A.T., Leisch H., et al. Unexpected reactivity of the burgess reagent with thiols:synthesis of symmetrical disulfides. J. Org. Chem. 72 (2007) 4989–4992. DOI:10.1021/jo070099t |
| [19] | Nair V., Augustine A.. Novel synthesis of 2-arylbenzothiazoles mediated by ceric ammonium nitrate (CAN):a rebuttal. Org. Lett. 5 (2003) 543–544. DOI:10.1021/ol027452w |
| [20] | Leino R., Lonnqvist J.E.. A very simple method for the preparation of symmetrical disulfides. Tetrahedron Lett. 45 (2004) 8489–8491. DOI:10.1016/j.tetlet.2004.09.100 |
| [21] | Patel S., Mishra B.K.. Cetyltrimethylammonium dichromate:a mild oxidant for coupling amines and thiols. Tetrahedron Lett. 45 (2004) 1371–1372. DOI:10.1016/j.tetlet.2003.12.068 |
| [22] | Joshi G., Bhadra S., Ghosh S., et al. Making full use of the oxidizing equivalents in bromate in the selective oxidation of thiols, sulfides, and benzylic/secondary alcohols into disulfides, sulfoxides, and aldehydes/ketones. Ind. Eng. Chem. Res. 49 (2010) 1236–1241. DOI:10.1021/ie901426t |
| [23] | Iranpoor N., Firouzabadi H., Pourali A.R.. Dinitrogen tetroxide supported on polyvinylpyrrolidone (PVP-N2O4):a new nitrosating and coupling agent for thiols and a selective oxidant for sulfides and disulfides. Tetrahedron 58 (2002) 5179–5184. DOI:10.1016/S0040-4020(02)00390-3 |
| [24] |
(a) K.Y.D. Tan, J.W. Kee, W.Y. Fan, CpMn(CO)3-catalyzed photoconversion of thiols into disulfides and dihydrogen, Organometallics 29(2010) 4459-4463; (b) A. Talla, B. Driessen, N.J.W. Straathof, et al., Metal-free photocatalytic aerobic oxidation of thiols to disulfides in batch and continuous-flow, Adv. Synth. Catal. 357(2015) 2180-2186; (c) P. Kumar, G. Singh, D. Tripathi, et al., Visible light driven photocatalytic oxidation of thiols to disulfides using iron phthalocyanine immobilized on graphene oxide as a catalyst under alkali free conditions, RSC Adv. 4(2014) 50331-50337; (d) X.B. Li, Z.J. Li, Y.J. Gao, et al., Mechanistic insights into the interface-directed transformation of thiols into disulfides and molecular hydrogen by visible-light irradiation of quantum dots, Angew. Chem. Int. Ed. 53(2014) 2085-2089; (e) S.S. Shah, S. Karthik, N.D.P. Singh, Vis/Nir light driven mild and clean synthesis of disulfides in the presence of Cu2(OH)PO4 under aerobic conditions, RSC Adv. 5(2015) 45416-45419; (f) M. Oba, K. Tanaka, K. Nishiyama, et al., Aerobic oxidation of thiols to disulfides catalyzed by diaryl tellurides under photosensitized conditions, J. Org. chem. 76(2011) 4173-4177. |
| [25] |
(a) A. Shojaei, M.A. Rezvani, M. Heravi, H5PV2Mo10O40 as an efficient catalyst for the oxidation of thiols to the corresponding disulfides using hydrogen peroxide as the oxidant, J. Serb. Chem. Soc. 76(2011) 955-963; (b) A. Ghorbani-Choghamarani, M. Nikoorazm, G. Azadi, In situ generated hypoiodous acid in an efficient and heterogeneous catalytic system for the homo-oxidative coupling of thiols, J. Serb. Chem. Soc. 78(2013) 173-178; (c) F. Rajabi, T. Kakeshpour, M.R. Saidi, Supported iron oxide nanoparticles:recoverable and efficient catalyst for oxidative S-S coupling of thiols to disulfides, Catal. Commun. 40(2013) 13-17. |
| [26] |
(a) P.J. Chai, Y.S. Li, C.X. Tan, An efficient and convenient method for preparation of disulfides from thiols using air as oxidant catalyzed by Co-salophen, Chin. Chem. Lett. 22(2011) 1403-1406; (b) A. Dhakshinamoorthy, M. Alvaro, H. Garcia, Aerobic oxidation of thiols to disulfides using iron metal-organic frameworks as solid redox catalysts, Chem. Commun. 46(2010) 6476-6478; (c) A. Corma, T. Rodenas, M.J. Sabater, Aerobic oxidation of thiols to disulfides by heterogeneous gold catalysts, Chem. Sci. 3(2012) 398-404; (d) T.V. Rao, B. Sain, P.S. Murthy, et al., Iron (Ⅲ)-ethylenediaminetetraacetic acid mediated oxidation of thiols to disulfides with molecular oxygen, J. Chem. Res. (1997) 300-301; (e) R. Kumar, N. Sharma, U.K. Sharma, et al., First metal-and base-free selective oxidative coupling of thiols in neat ionic liquids:NMR probed "ambiphilic" character of neutral[Hmim]Br towards atom-efficient synthesis of disulfides, Adv. Synth. Catal. 354(2012) 2107-2112; (f) G. Singh, P.K. Khatri, S.K. Ganguly, et al., Magnetic silica beads functionalized with cobalt phthalocyanine for the oxidation of mercaptans in an alkali free aqueous medium, RSC Adv. 4(2014) 29124-29130; (g) A. Shard, R. Kumar, N. Sharma, et al., Amino acid and water-driven tunable green protocol to access S-S/C-S bonds via aerobic oxidative coupling and hydrothiolation, RSC Adv. 4(2014) 33399-33407; (h) P. Das, S. Ray, A. Bhaumik, et al., Cubic Ag2O nanoparticle incorporated mesoporous silica with large bottle-neck like mesopores for the aerobic oxidative synthesis of disulfide, RSC Adv. 5(2015) 6323-6331; (i) S. Thurow, V.A. Pereira, D.M. Martinez, et al., Base-free oxidation of thiols to disulfides using selenium ionic liquid, Tetrahedron Lett. 52(2011) 640-643; (j) D. Singh, F.Z. Galetto, L.C. Soares, et al., Metal-free air oxidation of thiols in recyclable ionic liquid:a simple and efficient method for the synthesis of disulfides, Eur. J. Org. Chem. (2010) 2661-2665; (k) N. Iranpoor, H. Firouzabadi, A.R. Pourali, Dinitrogen tetroxide impregnated charcoal (N2O4/Charcoal):selective oxidation of thiols to disulfides or thiosulfonates, Phosphorus Sulfur Silicon Realt. Elem. 181(2006) 473-479; (l) A. Hadi, S.H. Allah, I. Nasser, Iron(Ⅲ) trifluoroacetate:chemoselective and recyclable lewis and transthioacetalization of carbonyl compounds and aerobic coupling of thiols, Chin. J. Chem. 26(2008) 2086-2092. |
| [27] | Csende F.. Alkyl nitrites as valuable reagents in organic synthesis. Mini-Rev. Org. Chem. 12 (2015) 127–148. DOI:10.2174/1570193X1202150225152405 |
| [28] |
(a) J.Q. Ma, Z.M. Hu, M.C. Li, et al., DDQ/tert-Butyl nitrite-catalyzed aerobic oxidation of diarylmethane sp3 C-H bonds, Tetrahedron 71(2015) 6733-6739; (b) Z.L. Shen, M. Chen, T.T. Fang, et al., Transformation of ethers into aldehydes or ketones:acatalytic aerobic deprotection/oxidation pathway, Tetrahedron Lett. 56(2013) 2768-2772; (c) Z.L. Shen, L.L. Sheng, X.C. Zhang, et al., Aerobic oxidative deprotection of benzyl-type ethers under atmospheric pressure catalyzed by 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ)/tert-butyl nitrite, Tetrahedron Lett. 54(2013) 1579-1583; (d) C.B. Qiu, L.Q. Jin, Z.L. Huang, et al., Symbiotic catalysis relay:molecular oxygen activation catalyzed by multiple small molecules at ambient temperature and its mechanism, ChemCatChem 4(2012) 76-80; (e) Z.L. Shen, J.L. Dai, J. Xiong, et al., 2, 3-Dichloro-5, 6-dicyano-1, 4-benzoquinone (DDQ)/tert-butyl nitrite/oxygen:a versatile catalytic oxidation system, Adv. Synth. Catal. 353(2011) 3031-3038; (f) X.J. He, Z.L. Shen, W.M. Mo, et al., TEMPO-tert-butyl nitrite:an efficient catalytic system for aerobic oxidation of alcohols, Adv. Synth. Catal. 351(2009) 89-92. |
| [29] | Bellale E.V., Chaudhari M.K., Akamanchi K.G.. A simple, fast and chemoselective method for the preparation of arylthiols. Synthesis (2009) 3211–3213. |
| [30] |
(a) A. Keszler, Y.H. Zhang, N. Hogg, Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein:How are S-nitrosothiols formed? Free Radical Biol. Med. 48(2010) 55-64; (b) M.A. Zolfigol, Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions, Tetrahedron 57(2001) 9509-9511; (c) L. Grossi, P.C. Montevecchi, S-nitrosocysteine and cystine from reaction of cysteinewithnitrousacid.AkineticInvestigation, J.Org.Chem.67(2002)8625-8630. |
 2016, Vol. 27
2016, Vol. 27