b Graduate University of Chinese Academy of Sciences, Bejing 100039, China ;
c State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China
Cyclohexanone, which was produced by oxidation of cyclohexane or the hydrogenation of phenol, is a key intermediate for the manufacture of both caprolactam for Nylon 6 and adipic acid for Nylon 66 [1-5]. Oxidation of cyclohexane requires high temperatures (140-180 ℃) and high pressures (8-20 bar), and generates some byproducts such as cyclohexanol and cyclohexyl hydroperoxide [6]. The phenol hydrogenation route can be generated cyclohexanone much more convenient and efficient in a "one-step" or a "two-step" process, in which the "two-step" process involves hydrogenated phenol to cyclohexanol [7], and the production dehydrogenates to cyclohexanone at high temperatures [8, 9]. In contrast, the direct "one-step" hydrogenation of phenol to cyclohexanone process is more advantageous and identified as an atom economic method in which the endothermic step of cyclohexanol dehydrogenation can be avoided [2, 3, 10].
Some catalysts have been evaluated in the one-step hydrogenation of phenol, and the results showed that the selectivity of cyclohexanone is largely dependent on the catalyst properties [10-17]. Rode et al. [18] used supercritical CO2 as a solvent, which requires high H2 and CO2 pressures (>7.0 MPa). Han et al. [19] reported that dual-supported Pd Lewis acid catalyst was a selective and efficient catalyst, the AlCl3 assisted activation of the phenyl ring, and the prevention of further hydrogenation to cyclohexanol, which imposes severe limitations on the applications of hydrogenation in general and adds a chemical sensitivity that restricts substrates, purity, and reaction conditions. Recently, Wang et al. [20] described that Pd nanoparticles (PdNPs) supported on a mpgC3N4, is an efficient and selective catalyst in hydrogenation of phenol in an aqueous media. However, the preparation of the mpgC3N4 support involved the use of aqueous ammonium bifluoride (NH4HF2) and/or hydrogen fluoride (HF) which are hazardous and not environmentally friendly. Therefore, it is desirable to search for an alternative heterogeneous catalyst for selective hydrogenation of phenol under ambient conditions with high activity and selectivity.
Tungsten carbides have been reported as efficient supports for the preparation of platinum-based electrocatalysts [21]. The role of semiconducting supports in heterogeneous catalysts and their ability to modify the electron density of the supported metal thereby modifying their activities has been highlighted [22]. TiN is a biocompatible material with excellent physical and chemical properties including thermal stability and acid resistance [23, 24]. Thus, it could be applied as support for preparing nanoscale catalysts. Ni/TiN catalysts for the hydrogenolysis of aryl ethers as models for lignin [25] have been reported. The close contact between TiN and Ni was observed by electron transfer from Ni to TiN, and the electron transfer promoted the catalytic activity of Ni. In this work, a new method using Pd/TiN catalysts for the synthesis of cyclohexanone by the selective hydrogenation of phenol were described. It was found that TiN as support enhanced the catalytic activity of PdNPs.
2. Experimental 2.1. Materials and instrumentsTiN was purchased from Hefei Kaier Nanometer Energy & Technology Co., Ltd. 5.0 wt% Pd/C was purchased from Shanxi Kaida Chemical Engineering Co., Ltd. All other chemical reagents were purchased from Kailaiying Medical Chemical (Tianjin) Co., Ltd. The morphology and the particle size distribution of the TiN supported PdNPs were determined by a JEM-2010 TEM with an accelerating voltage of 200 kV. Powder X-ray diffraction (XRD) measurements were performed on an X’Pert Pro multipurpose diffractometer (PANalytical, Inc.) with Nifiltered Cu Kα radiation (0.15046 nm) at room temperature from 10.0° to 80.0° (wide angle) and 0.6° to 5° (small angle). X-ray photoelectron spectra (XPS) analyses of the catalysts were performed on a Thermo Fisher Scientific KAlpha spectrometer.
2.2. Fabrication of Pd/TiN catalystsAn aqueous solution of H2PdCl4 was initially prepared by mixing of PdCl2 into 10% (v/v) HCl aqueous solution by stirring at room temperature until the salt was homogeneously dissolved. A certain amount of the TiN powder was impregnated with the H2PdCl4 solution and stirred at room temperature for 8 h. Then excess NaBH4 aqueous solution was slowly added to such a suspension mixture under ice bath conditions. The corresponding supported PdNPs were separated by centrifugation, washed sequentially using with distilled water and absolute ethanol several times, and dried at 60 ℃ overnight in a vacuum oven to give expected Pd/TiN catalysts.
2.3. Hydrogenation of phenolA typical procedure for the hydrogenation of phenol was as follows: phenol (47 mg, 0.5 mmol), Pd/TiN (2.5-5 mol%) and H2O (2 mL), CH2Cl2 (1 mL), were placed into a 50 mL stainless steel autoclave. The reactor system was purged with N2 three times followed by H2 three times. The autoclave was pressurized with 0.2 MPa of H2. The reaction mixture was stirred vigorously at the desired reaction temperature. After a prescribed reaction time, the autoclave was cooled to room temperature and the residue gas was released. The catalyst was removed from the liquid by filtration, and then the organic phase was extracted. The conversion and selectivity were determined by a GC 112A equipped with a FID detector and an SE-54 column (30 m × 0.25 mm × 0.25 mm film thickness).
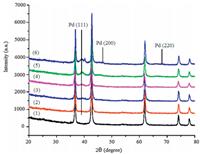
3. Results and discussion 3.1. The XRD patterns of the Pd/TiN catalystsFig. 1 shows the XRD patterns of the Pd/TiN catalysts. As can be seen, the diffraction peak at 36.1° in the XRD patterns can be assigned to the TiN (1 1 1) [23] (Fig. 1, curve 1). The diffraction peaks at 40.1°, 46.7°, and 68.1° can be assigned to the (1 1 1), (2 0 0), (2 2 0) planes of the face centered cubic Pd particles [26], respectively. The patterns of Pd/TiN1.3, Pd/TiN2.5, and Pd/TiN5 only show (1 1 1) diffractions, other diffraction peaks of the Pd are not observed at all (Fig. 1, curves 2-4). Although the Pd loading content for Pd/TiN5 is as high as 5.0 wt%, the PdNPs are still not detected by XRD, which possibly indicates that the supported PdNPs have small particle sizes and are homogeneously dispersed on the surface of the TiN. The diffraction peaks showed no significant changes for the used four reaction cycles of Pd/TiN5 (Fig. 1, curve 5). The results indicated that TiN can efficiently stabilize the PdNPs catalysts in the selective hydrogenation of phenol. However, with the Pd loadings increasing, the characteristic diffraction peaks of the Pd were obviously found (Fig. 1, curve 6).
|
Download:
|
| Figure 1. XRD patterns of Pd/TiN with different Pd loadings. (1) TiN; (2) Pd/TiN1.3; (3) Pd/TiN2.5; (4) Pd/TiN5; (5) Pd/TiN5 (after four reaction cycles); (6) Pd/TiN8.5. | |
3.2. The TEM images of Pd/TiN catalysts
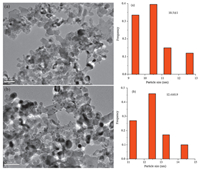
The TEM images shows the morphology and the particle size distribution of the TiN supported PdNPs prepared at four kinds of catalysts with different Pd loadings Pd/TiN5 (Fig. 2a) and Pd/TiNx (x=1.3, 2.5, 8.5, x represented wt% Pd loadings) (Fig. S1 in Supporting information). These images reveal well-dispersed Pd particles and no agglomeration. The average particle sizes of Pd/ TiN1.3 (Fig. S1a), Pd/TiN2.5 (Fig. S1b) and Pd/TiN5 (Fig. 2a) are 7.5±1.1 nm, 7.7±0.9 nm and 10.5±1 nm, respectively. It is most likely that the TiN supports play a key role in preventing the uncontrollable growth of the PdNPs and stabilizing the formed PdNPs effectively. However, as shown in Fig. 2b, the PdNPs particle size increased slightly after four reaction cycles of Pd/TiN5 and the catalytic activity of these PdNPs correspondingly decreased in the selective hydrogenation of phenol. The Pd particle sizes obviously increased to 16.5 nm with the increasing Pd loading to 8.5 wt% (Fig. S1c). The corresponding mean particle diameters were measured and calculated by counting 200 particles from the enlarged photographs.
|
Download:
|
| Figure 2. TEM images and the particle size distribution of (a) Pd/TiN5 and (b) Pd/TiN5 (after four reaction cycles). | |
3.3. The XPS characterization of Pd/TiN5
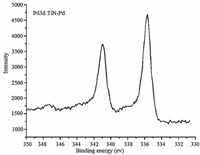
The Pd/TiN5 was characterized by XPS. The results are shown in Fig. 3. The spectra present a doublet corresponding to Pd 3d5/2 and 3d3/2. The Pd 3d5/2 peak at 335.6 eV and 3d3/2 peak at 340.9 eV is attributed to Pd0 (metallic palladium), which shows that nearly all of the Pd2+ ions were reduced, and Pd0 was the only form in the PdNPs prepared by NaBH4 [26].
|
Download:
|
| Figure 3. XPS spectra of Pd/TiN5. | |
3.4. Hydrogenation
Table 1 presents the results of phenol hydrogenation under different conditions over the Pd/TiN catalysts. The four kinds of catalysts synthesized by varying the Pd loadings from 1.3 wt% to 8.5 wt% Pd/TiNx (x=1.3, 2.5, 5.0, 8.5) were evaluated. It can be seen that as Pd loadings were increased from 1.3 wt% to 5.0 wt%, the conversion and the selectivity were also gradually increased correspondingly, and the conversions of phenol were 44%, 45%, and 49% with the selectivity of cyclohexanone were 92%, 92%, and 97% under 0.2 MPa of H2 at 60 ℃ for 2 h (Table 1, entries 1-3), respectively. These results may be due to the fact that the same amount of reactive Pd sites, the content of TiN was different, and the residual TiN partially influences catalytic activity by hindering access of reactants to active Pd sites. However, when the amount of Pd loading was increased from 5.0% to 8.5 wt%, the selectivity of cyclohexanone is still maintained at around 96%, but the conversion of phenol declined drastically to 28% (Table 1, entry 4). The reason for such a phenomenon can be attributed to the PdNPs was not stable and tend to aggregate slightly when the particle size of Pd was increased to about 16.5 nm as the amount of Pd loading was increased. These results may depend sensitively weak on the sizes of the Pd particles [27, 28]. When using Pd/TiN5 as a catalyst, the excellent conversion of 99% and selectivity of 98% was obtained (Table 1, entry 8), and the catalyst Pd/TiN5 was chosen for the investigation of reaction parameters in the hydrogenation of phenol.
|
|
Table 1 Hydrogenation of phenol with various Pd/TiN catalysts. |
The mixed solvents of H2O and CH2Cl2 were used in the selective hydrogenation of phenol and the effect of different solvent ratio on the conversion and selectivity was investigated (Table 1, entries 5-7). Initially, 2 mL of H2O and 3 mL CH2Cl2 was selected as mixture solvents and the conversion of phenol was only 10%, while the selectivity of cyclohexanone up to more than 99% (Table 1, entry 5). The conversion of phenol 26% and a declined selectivity of cyclohexanone 82% were obtained when increased the H2O to 3 mL and decreased CH2Cl2 to 1 mL (Table 1, entry 6). The volumes of H2O and CH2Cl2 were further adjusted to 1 mL and 2 mL, respectively, the conversion did not changed obviously, while the selectivity of cyclohexanone was rebounded to 97% (Table 1, entry 7). The above results showed that H2O was beneficial to the conversion of water soluble phenol, while CH2Cl2 was beneficial to the selectivity of oil soluble cyclohexanone. And when the mixed solvents of H2O (2 mL) and CH2Cl2 (1 mL) were selected as mixture solvents (Table 1, entry 8), the conversion and selectivity were up to 99% and 98%, respectively. It was observed that the higher temperature will accelerate the hydrogenation of phenol, for example, with increasing the reaction temperature from 60 ℃ to 100 ℃, the reaction time was shortened obviously, while the conversion and selectivity still remained (Table 1, entry 9). Furthermore, the conversion of phenol was increased gradually from 92% to 99% on prolonging the reaction time from 10 h to 12 h at 30 ℃ (Table 1, entries 11 and 12). In order to compare the selectivity of catalysts, 5.0 wt% Pd/C was used in the hydrogenation of phenol under otherwise identical conditions and only 60% selectivity of cyclohexanone was obtained (Table 1, entry 10). The TiN showed no catalytic activity in the absence of the PdNPs (Table 1, entry 13). These results revealed that the PdNPs are efficient and highly selectivity catalysts for the hydrogenation of phenol to cyclohexanone, which provided a new route for the fabrication of cyclohexanone via selective hydrogenation of phenol.
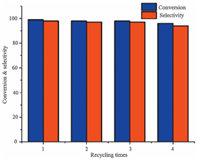
To illustrate the general applicability of Pd/TiN5, other phenol derivatives were investigated. Table 2 shows the results of the hydrogenation. It can be seen that 99% conversion was achieved in most cases, and the overall selectivity of cycloketones exceeded 93% in all cases. The conversions of p-cresol, o-cresol and m-cresol were all 99% (Table 2, entries 1-3). Hydrogenation of hydroquinone yielded the synthetically very valuable hydroxylcyclohexanones with high conversions and selectivities (Table 2, entry 4). However, the conversion of p-tert-butylphenol was only 90% at 100 ℃ for 20 h (Table 2, entry 5). When the substituents on the aromatic ring were changed, the conversion of the substrates and the selectivity of the products were changed accordingly. Methylphenol derivatives gave methylcyclohexanones with excellent conversion and selectivity, while p-tert-butylphenol showed a lower conversion and the selectivity (95%). This may be due to the bulky functional group inhibiting an attack of a palladium hydride species towards the phenol ring [29]. 3.5. The recyclability of Pd/TiN5 The recyclability of catalyst Pd/TiN5 was investigated in the selective hydrogenation of phenol in the mixed solvents of H2O and CH2Cl2. The recycling data were showed in Fig. 4. It was found that the catalyst Pd/TiN5 exhibited significantly high catalytic activity and selectivity for the hydrogenation of phenol and the selectivity of cyclohexanone only dropped from 98% to 94% after four reaction runs. The results also demonstrated that the PdNPs catalyst have an excellent stability. From the TEM images (Fig. 2b) of recycled catalyst, it can be observed that the morphology of recycled catalyst showed no obvious changes compared to fresh catalyst, except that growth or aggregation and the Pd/TiN5 particle size increased from 10.5±1 nm to 12.4±0.9 nm slightly after four reaction cycles. From the above result, it can be concluded that the catalytic activity of the catalyst decreased with the increase of the particle sizes of Pd/TiN5 in the selective hydrogenation of phenol.
|
|
Table 2 Hydrogenation of phenol derivatives using Pd/TiN5 catalyst in mixed solvent of H2O and CH2Cl2. |
|
Download:
|
| Figure 4. The recyclability of Pd/TiN5 catalyst. (Reaction condition: phenol (47 mg, 0.5 mmol), Pd/TiN5 (5 mol% relative to phenol), 0.2 MPa H2, H2O (2 mL), CH2Cl2 (1 mL), 30 ℃, 12 h.) | |
4. Conclusion
In conclusion, Pd/TiN with different size of Pd particles was fabricated by the chemical reduction with different Pd contents (1.3-8.5 wt%) and successfully applied in the hydrogenation of phenol and its derivatives. As a result of the uniform distribution of Pd particles, the catalytic activity and the combined selectivity to cyclohexanone increased consistently with the increasing Pd particle size to reach maximum values at around 10 nm in the range of 7-16.5 nm, which shows the structure sensitivity weak of the reaction. Pd/TiN5 exhibited high activity for the hydrogenation of phenol, p-cresol, o-cresol and m-cresol to the corresponding cyclohexanone in the mixed solvents of H2O and CH2Cl2 under mild conditions. However, the conversion was lower in the hydrogenation of p-tert-butylphenol. A detailed investigation of the reaction mechanism including the influence of other solvents and how to improve the catalytic activity for the hydrogenation of phenol derivatives will be the subject of future studies.
AcknowledgmentWe are grateful to the financial supports granted by the National Natural Science Foundation of China (No. 21174155).
| [1] | Kuklin S., Maximov A., Zolotukhina A., Karakhanov E.. New approach for highly selective hydrogenation of phenol to cyclohexanone:combination of rhodium nanoparticles and cyclodextrins. Catal. Commun. 73 (2016) 63–68. DOI:10.1016/j.catcom.2015.10.005 |
| [2] | Jiang H., Qu Z.Y., Li Y., et al. One-step semi-continuous cyclohexanone production via hydrogenation of phenol in a submerged ceramic membrane reactor. Chem. Eng. J. 284 (2016) 724–732. DOI:10.1016/j.cej.2015.09.037 |
| [3] | Chen J.Z., Zhang W., Chen L.M., et al. Direct selective hydrogenation of phenol and derivatives over polyaniline-functionalized carbon-nanotube-supported palladium. Chempluschem 78 (2013) 142–148. DOI:10.1002/cplu.v78.2 |
| [4] | Shore S.G., Ding E., Park C., Keane M.A.. The application of {(DMF)(10)Yb-2[TM(CN)(4)](3)}(infinity) (TM=Ni Pd) supported on silica to promote gas phase phenol hydrogenation. J. Mol. Catal. A:Chem. 212 (2004) 291–300. DOI:10.1016/j.molcata.2003.11.004 |
| [5] | Wang Y., Zhang J.S., Wang X.C., Antonietti M., Li H.R.. Boron-and fluorinecontaining mesoporous carbon nitride polymers:metal-free catalysts for cyclohexane oxidation. Angew. Chem. Int. Ed. 49 (2010) 3356–3359. DOI:10.1002/anie.201000120 |
| [6] | Nelson N.C., Manzano J.S., Sadow A.D., Overbury S.H., Sowing I.I.. Selective hydrogenation of phenol catalyzed by palladium on high-surface-area ceria at room temperature and ambient pressure. ACS Catal. 5 (2015) 2051–2061. DOI:10.1021/cs502000j |
| [7] | Fridman V.Z., Davydov A.A.. Dehydrogenation of cyclohexanol on copper-containing catalysts I. The influence of the oxidation state of copper on the activity of copper sites. J. Catal. 195 (2000) 20–30. DOI:10.1006/jcat.2000.2979 |
| [8] | Zhang F.W., Chen S., Li H., Zhang X.M., Yang H.Q.. Pd nanoparticles embedded in the outershell of a mesoporous core-shell catalyst for phenol hydrogenation in pure water. RSC Adv. 5 (2015) 102811–102817. DOI:10.1039/C5RA12947H |
| [9] | Chatterjee M., Kawanami H., Sato M., et al. Hydrogenation of phenol in supercritical carbon dioxide catalyzed by palladium supported on Al-MCM-41:a facile route for one-pot cyclohexanone formation. Adv. Synth. Catal. 351 (2009) 1912–1924. DOI:10.1002/adsc.v351:11/12 |
| [10] | Ertas I.E., Gulcan M., Bulut A., Yurderi M., Zahmakiran M.. Rhodium nanoparticles stabilized by sulfonic acid functionalized metal-organic framework for the selective hydrogenation of phenol to cyclohexanone. J. Mol. Catal. A:Chem. 410 (2015) 209–220. DOI:10.1016/j.molcata.2015.09.025 |
| [11] | Shore S.G., Ding E.R., Park C., Keane M.A.. Vapor phase hydrogenation of phenol over silica supported Pd and Pd-Yb catalysts. Catal. Commun. 3 (2002) 77–84. DOI:10.1016/S1566-7367(02)00052-3 |
| [12] | Xiang Y.Z., Kong L.N., Lu C.S., Ma L., Li X.N.. Lanthanum-promoted Pd/Al2O3 catalysts for liquid phase in situ hydrogenation of phenol to cyclohexanone. React. Kinet. Mech. Cat. 100 (2010) 227–235. |
| [13] | Scire S., Minico S., Crisafulli C.. Selective hydrogenation of phenol to cyclohexanone over supported Pd and Pd-Ca catalysts:an investigation on the influence of different supports and Pd precursors. Appl. Catal. A:Gen. 235 (2002) 21–31. DOI:10.1016/S0926-860X(02)00237-5 |
| [14] | Chen Y.Z., Liaw C.W., Lee L.I.. Selective hydrogenation of phenol to cyclohexanone over palladium supported on calcined Mg/Al hydrotalcite. Appl. Catal. A:Gen. 177 (1999) 1–8. DOI:10.1016/S0926-860X(98)00252-X |
| [15] | Neri G., Visco A.M., Donato A., et al. Hydrogenation of phenol to cyclohexanone over palladium and alkali-doped palladium catalysts. Appl. Catal. A:Gen. 110 (1994) 49–59. DOI:10.1016/0926-860X(94)80104-5 |
| [16] | Wang Y., Yao J., Li H.R., Su D.S., Antonietti M.. Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media. J. Am. Chem. Soc. 133 (2011) 2362–2365. DOI:10.1021/ja109856y |
| [17] | Liu H.Z., Jiang T., Han B.X., Liang S.G., Zhou Y.X.. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-lewis acid catalyst. Science 326 (2009) 1250–1252. DOI:10.1126/science.1179713 |
| [18] | Rode C.V., Joshi U.D., Sato O., Shirai M.. Catalytic ring hydrogenation of phenol under supercritical carbon dioxide. Chem. Commun. (2003) 1960–1961. |
| [19] | Liu H., Jiang T., Han B., Liang S., Zhou Y.. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst. Science 326 (2009) 1250–1252. DOI:10.1126/science.1179713 |
| [20] | Wang Y., Yao J., Li H., Su D., Antonietti M.. Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media. J. Am. Chem. Soc. 133 (2011) 2362–2365. DOI:10.1021/ja109856y |
| [21] | Esposito D.V., Hunt S.T., Kimmel Y.C., Chen J.G.G.. A new class of electrocatalysts for hydrogen production from water electrolysis:metal monolayers supported on low-cost transition metal carbides. J. Am. Chem. Soc. 134 (2012) 3025–3033. DOI:10.1021/ja208656v |
| [22] | Li X.H., Antonietti M.. Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides:functional Mott-Schottky heterojunctions for catalysis. Chem. Soc. Rev. 42 (2013) 6593–6604. DOI:10.1039/c3cs60067j |
| [23] | Hammerle H., Kobuch K., Kohler K., et al. Biostability of micro-photodiode arrays for subretinal implantation. Biomaterials 23 (2002) 797–804. DOI:10.1016/S0142-9612(01)00185-5 |
| [24] | Marlo M., Milman V.. Density-functional study of bulk and surface properties of titanium nitride using different exchange-correlation functionals. Phys. Rev. B 62 (2000) 2899–2907. DOI:10.1103/PhysRevB.62.2899 |
| [25] | Molinari V., Giordano C., Antonietti M., Esposito D.. Titanium nitride-nickel nanocomposite as heterogeneous catalyst for the hydrogenolysis of aryl ethers. J. Am. Chem. Soc. 136 (2014) 1758–1761. DOI:10.1021/ja4119412 |
| [26] | Zhu J.F., Tao G.H., Liu H.Y., et al. Aqueous-phase selective hydrogenation of phenol to cyclohexanone over soluble Pd nanoparticles. Green Chem. 16 (2014) 2664–2669. DOI:10.1039/c3gc42408a |
| [27] | Shuai D., McCalman D.C., Choe J.K., et al. Structure sensitivity study of waterborne contaminant hydrogenation using shape-and size-controlled Pd nanoparticles. ACS Catal. 3 (2013) 453–463. DOI:10.1021/cs300616d |
| [28] | Crespo-Quesada M., Yarulin A., Jin M., Xia Y., Kiwi-Minsker L.. Structure sensitivity of alkynol hydrogenation on shape-and size-controlled palladium nanocrystals:which sites are most active and selective?. J. Am. Chem. Soc. 133 (2011) 12787–12794. DOI:10.1021/ja204557m |
| [29] | Chen A., Zhao G., Chen J., Chen L., Yu Y.. Selective hydrogenation of phenol and derivatives over an ionic liquid-like copolymer stabilized palladium catalyst in aqueous media. RSC Adv. 3 (2013) 4171–4175. DOI:10.1039/c3ra21663b |
 2016, Vol. 27
2016, Vol. 27