Exposure to ultraviolet (UV) rays (λ=200-400 nm) can produce accelerated photodegradation of organic polymers, pigments, and dyes. Furthermore, such rays are harmful to human health causing sunburn, photoaging and skin cancer [1, 2]. Consequently, protection against human exposure to UV radiation has attracted significant interest [3]. Thus far, most UV absorbers are organic materials which have excellent UV absorption ability. However, some disadvantages restrict their wide application, such as instability and toxicity at high concentration [4]. Therefore, any alternative attempts to incorporate the organic UV absorber into an inorganic host matrix to avoid direct contact of organic molecules with the human skin would be beneficial. The supramolecular layered, host-guest structure enables both physical shielding and absorption of UV light. Furthermore, incorporating an organic absorber in an inorganic host should increase its thermal and photostability [5, 6].
The empirical composition of layered double hydroxides (LDHs) can be represented by the general formula: [M1-xⅡMxⅢ(OH)2] (An-)x/n·mH2O, where MⅡ and MⅢ are divalent and trivalent metals, respectively; (An- represents inter-lamellar anions that compensate for the positive charge of the layer; m is the moles of water molecules located in the interlayer galleries together with the anions [7-9]. A wide range of tunable metal ions have been extensively explored in the fields of catalysis, separation, biology, drugs and functional additives [10-14] without changing the structure of the material and anion exchange properties of LDH materials. Layered inorganic compounds (clays, layered double hydroxides and others) can absorb, scatter and reflect the UV radiation. Intercalation of some UV absorbers into the interlayer galleries of LDHs has previously been reported. However, the intercalation of UV absorber into ZnTi-LDH is rare/uncommon. In addition to the typical divalent and trivalent cations, the combination of di-and tetra-valent metal ions has also been shown to be feasible (e.g., Sn4+, Ti4+, etc.) [15, 16]. As is well known, titanium dioxide (TiO2) and zinc oxide (ZnO) minerals are frequently employed as inorganic physical sun blockers and while TiO2 is more effective in UVB and ZnO in the UVA range, the synthesis of ZnTi-LDH can achieve the effect of combination of these metals to assure broad-band UV protection. The ZnTi-LDH combination must exhibit a stronger anti-ultraviolet ability than many LDHs with other metal ions (e.g., MgAl-LDH, ZnAl-LDH, etc.).
In this work, p-aminobenzoic acid (PABA) was intercalated into ZnTi-LDH by an anion-exchange reaction. PABA is a UV-B absorbent used as a sunscreen component and it tends to give rise to adverse cutaneous reactions like irritation, contact orticaria and allergic and photoallergic dermatitis [17-19]. The chemical structure of PABA is shown in Fig. 1. Luana Perioli et al. [20], studied the safety and photostability properties of ZnAl-LDH, MgAl-LDH intercalated with PABA. Their results indicated that the nanocomposites obtained after intercalation had better UV absorption ability and enhanced the safety of PABA. We have successfully prepared the ZnTi-LDH precursor initially, As a result of the shielding effect of the LDH layers and the incorporation of titanium element in the layers, ZnTi-LDH exhibits a stronger anti-ultraviolet ability. The supramolecular layered host-guest structure of ZnTiPABA-LDH was characterized by XRD, FT-IR, TG-DTA. The UV ray resistance ability and photocatalytic capability were investigated.

|
Download:
|
| Figure 1. Chemical structural formula of p-aminobenzoic acid. | |
2. Experimental 2.1. Materials
Analytical grade Zn(NO3)2·6H2O, TiCl4, urea and PABA were purchased from Sinopharm Chemical Reagent Co., Ltd. and used without further purification. The deionized and decarbonated water was used in all the preparation processes.
2.2. Preparation of ZnTi-LDHThe ZnTi-LDH precursor was prepared by the co-precipitation of zinc and titanium salts from a homogeneous solution. TiCl4 (0.44 mL), Zn(NO3)2·6H2O (4.76 g) and urea (6.0 g) were dissolved in deionized water (200 mL) under vigorous stirring. The resulting reactant was aged in an autoclave at 130 ℃ for 48 h. The precipitate was centrifuged, washed thoroughly with deionized water, and finally dried at 60 ℃ for 12 h.
2.3. Preparation of ZnTi-PABA-LDHThe PABA anion intercalated LDH (ZnTi-PABA-LDH) was prepared by anion-exchange using ZnTi-LDH precursor. PABA (0.2 mol L-1) was dissolved by ultrasonic treatment in CO2-free deionized water to form an aqueous solution. The ZnTi-LDH precursor (0.12 mol L-1) was mixed with the solution of PABA in CO2-free deionized water followed by aging under N2 atmosphere at 60 ℃ overnight. The resulting precipitate was centrifuged, thoroughly washed, and dried under vacuum at 60 ℃ overnight.
2.4. Characterization of samplesThe X-ray diffraction (XRD) patterns of the samples were recorded on an X-ray powder diffractometer (Bruker D2, Karlsruhe, Germany), using Cu Kα radiation (λ=1.5406 Å) at 30 kV, 10 mA, with a scanning rate of 5° min-1 in the 2θ range from 5° to 70°. The FT-IR spectra were recorded on a Thermo Scientific Nicolet IS 10 instrument in attenuated total reflectance (ATR) mode. Thermogravimetric (TG) analysis was performed on TGA Q5000IR instrument in the temperature range 20-650 ℃ with a heating rate of 10 ℃ min-1 in air. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4700 scanning electron microscope operating at 20 kV. High resolution TEM (HRTEM) observations were carried out on a JEOL JEM-2100 transmission electron microscope. Diffuse reflectance UV-vis-NIR spectra were recorded at r.t. in air on a Shimadzu UV-3700 spectrometer equipped with an integrating sphere attachment using BaSO4 as background. The generation of radical species was monitored by electron spin resonance (EPR) spectroscopy (Bruker E500 EPR spectrometer) with dimethylpyridine N-oxide (DMPO) as a spintrapping reagent. The samples are irradiated with a lamp simulating solar light for 1 min.
3. Results and discussion 3.1. Crystal structure and composition of ZnTi-LDH and ZnTi-PABALDHThe XRD patterns of ZnTi-LDH precursor and ZnTi-PABA-LDH are shown in Fig. 2. The XRD pattern of the ZnTi-LDH precursor (Fig. 2a) exhibits a strong d003 peak reflection at 13.22°. The ZnTiLDH also shows the reflections of (006), (009) at 24.42°, 35.02° [21], which can be indexed to typical LDH materials. The interlayer distance d003 is 0.669 nm. It should be noted that several weak reflections appear at 2θ=22°, 31° and 36° for ZnTi-LDH, which can be assigned to a small amount of zinc hydroxide impurity (PDF card No. 20-1437). In the case of ZnTi-PABA-LDH (Fig. 2b) the main characteristic reflections of the product appear at 9.68° (003), 16.08° (006), 19.25° (009). The basal spacing for ZnTi-PABA-LDH is 0.913 nm. The results indicated that the organic UV absorbent PABA were successfully intercalated into the interlayer spaces of ZnTi-LDH.
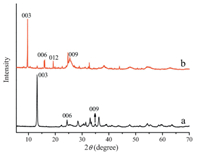
|
Download:
|
| Figure 2. XRD patterns of (a) ZnTi-LDH precursor and (b) ZnTi-PABA-LDH. | |

|
Download:
|
| Figure 3. FT-IR spectra of (a) ZnTi-LDH precursor, (b) PABA and (c) ZnTi-PABA-LDH. | |
The FT-IR spectra of ZnTi-LDH precursor, PABA and ZnTi-PABALDH are shown in Fig. 3. The spectrum of ZnTi-LDH precursor (Fig. 3a) shows a strong broad absorption band between 3500 and 3100 cm-1 due to the OH stretching mode of layer hydroxyl groups and of interlayer water molecules. The band at 1502 cm-1 together with its accompanying band at 1384 cm-1 is attributed to mode ν3 of interlayer carbonate species [22]. The FT-IR spectrum of PABA (Fig. 3b) showed two peaks around 3463 and 3363 cm-1 due to the amine group and a peak at 1665 cm-1 due to the carboxylic group. The spectrum of the ZnTi-PABA-LDH (Fig. 3c) showed a broad band between 3200 and 3700 cm-1, due to the stretches of hydrogenbonded hydroxyl group of both hydroxide layers and interlayer water. The stretching vibration of the carboxylic acid group moved from 1665 cm-1 to 1540 cm-1 and 1400 cm-1 (the antisymmetric and symmetric stretching bands) of the carboxylic group. The results indicate that the PABA anions have been successful intercalated into the interlayer galleries of the LDH. While the characteristic peak of the carbonate anion was absent, confirming that the carbonate groups have been completely displaced by the PABA anions.
3.2. Surface morphology of ZnTi-LDH and ZnTi-PABA-LDHThe surface morphology and particle size of ZnTi-LDH precursor and ZnTi-PABA-LDH were investigated by SEM and TEM. The synthesized Zn-Ti-LDH shows sheet-like morphology (Fig. 4A), with a diameter of about 300 nm-1 μm. From the SEM micrograph (Fig. 4B), it can be seen that the ZnTi-PABA-LDH particles are aggregated and clearly exhibited the presence of hierarchical structure with the sizes ranging between 3 μm and 8 μm. The HRTEM micrographs of ZnTi-LDH (Fig. 4C) and ZnTi-PABA-LDH (Fig. 4D) indicate the single-crystalline nature of the ZnTi-LDH material. The lattice with interplanar spacing of 0.38 nm and 0.47 nm was observed, corresponding to the (006) plane of ZnTiLDH phase and (012) of ZnTi-PABA-LDH. The value is in accordance with the in-plane structural parameter of ZnTi-LDH (d006=0.36 nm) and ZnTi-PABA-LDH (d009=0.48 nm) crystals determined from the XRD characterization.

|
Download:
|
| Figure 4. SEM images of (A) ZnTi-LDH precursor and (B) ZnTi-PABA-LDH; HRTEM images of (C) ZnTi-LDH precursor and (D) ZnTi-PABA-LDH. | |
3.3. UV absorption properties
The UV-vis diffuse reflectance spectra of ZnTi-LDH, PABA, and ZnTi-PABA-LDH are shown in Fig. 5. The spectrum of ZnTi-LDH shows strong UV absorption between 200 and 400 nm due to the shielding effect of the LDH layers as well as the incorporation of the titanium element in the layers. The absorption spectra of the free solid and intercalated PABA (Fig. 5b and c, repectively), are strongly affected by the microenvironment. The spectrum obtained for the pristine solid PABA presents UV absorption between 200 nm and 350 nm. The major absorption bands are observed at 340, 252 and 215 nm. The band at 340 nm is primarily due to a π-π* transition of the benzene ring. The band at 252 nm corresponds to a mixture of n-π* and π-π* transitions; it consists of a charge transfer from the carboxyl group and amino group toward the benzene ring. Similarly, the bands at 215 nm arise from a mixture of n-π* and π-π* transitions. After intercalation, ZnTi-PABA-LDH exhibits excellent UV absorption ability between 200 nm and 400 nm, stronger than both pristine PABA and ZnTi-LDH, which is attributed to the interactions between the guest and host layers [23, 24]. The broadening and enhancing of the absorption bands observed for ZnTi-PABA-LDH leads to an increase of the protection range for the intercalation compound compared to the pristine compound. Therefore, the establishment of these specific interactions between the sunscreen and the matrix is essential to extend its protection range.
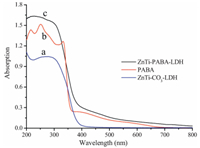
|
Download:
|
| Figure 5. UV–vis absorbance spectra of (a) ZnTi-LDH, (b) PABA and (c) ZnTi-PABA-LDH. | |
3.4. Thermal and photostability for ZnTi-PABA-LDH
Thermolysis behavior of both pristine and intercalated PABA was studied. As shown in Fig. 6A, DTA of PABA showed an endothermic peak at 191 ℃ relative to the melting point of the pure crystalline substance. In the thermograms of the intercalation compound, the lack of the endothermic peak at 191 ℃ is a confirmation that the UV absorbent intercalation process occurred, which is in accordance with the results of XRD and FT-IR. After intercalation of PABA into the LDH host, the thermal decomposition characteristics of the resulting product are significantly different. For the ZnTi-PABA-LDH, the DTA curve has an endothermic peak near 95 ℃ with a corresponding weight loss in the TG curve below 100 ℃. This is caused by the removal of interlayer water. With increasing temperature, there are three exothermic peaks at 411, 477 and 507 ℃, which can be attributed to the combustion of the organic guest, for which the corresponding TG curve shows a strong weight-loss stage. The results suggest that the thermal stability of PABA is enhanced by intercalation into the galleries of LDH. The TG-DTA data are consistent with the FT-IR data, which all suggest that ZnTi-PABA-LDH is a complex system of strong supramolecular interactions between host layers and guest and not a simple mixture of PABA and ZnTi-PABA-LDH. The interactions between host layers and guest anions involve electrostatic attraction between opposite charges, hydrogen bonding, and the van der Waals force.

|
Download:
|
| Figure 6. TG-DTA curves of (A) PABA and (B) ZnTi-PABA-LDH. | |
Both TiO2 and ZnO are often employed as ultraviolet blocking agents in sunscreens. However, as semiconductors, their highly photocatalytic activity is to facilitate the generation of active species, The strong oxidizing and reducing ability of these radical species both degrade the organic compounds in the matrix and damage the living organism [25, 26]. The photocatalytic performance of ZnTi-PABA-LDH with solar light response attracted our research interest. In order to understand the redox ability of the ZnTi-PABA-LDH, the spin-trapping electron spin resonance (ESR) technique was employed to monitor the presence of active radicals formed in the ZnTi-PABA-LDH.
Hydroxyl radicals (OH•) and superoxide anion radicals (O2•-) are the primary reactive oxygen species. The generation of active radicals (OH• and O2•-) from the ZnTi-PABA-LDH was confirmed by ESR with dimethylpyridine N-oxide (DMPO) as a spin-trapping reagent. The intense ESR signal observed for the sample corresponds to the adduct of the spin trap molecules with hydroxyl radicals (DMPO-OH•) and superoxide anion radicals (DMPO-O2•-). Before detecting by electron spin resonance (EPR) spectroscopy, the samples are irradiated with a lamp simulating solar light for 1 min. As shown in Fig. 7, the six typical peaks for DMPO-O2•- were observed in methanol. Besides, the three characteristic peaks for DMPO-OH• with intensity ratio of 1:2:2:1 were obviously observed in Fig. 8. The intensity of three samples correspond to the generation of active radicals (OH• and O2•-). Compared with TiO2 and ZnO, the ESR signal of ZnTi-LDH is remarkably weak. The results indicate that the ZnTi-PABA-LDH have a relatively lower photocatalytic activity (generate relatively lower active radicals (OH• and O2•-). Therefore, ZnTi-PABA-LDH is more suitable as a novel UV-shielding material.
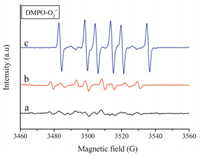
|
Download:
|
| Figure 7. DMPO spin-trapping ESR spectra recorded for (a) ZnTi-PABA-LDH, (b) TiO2 and (c) ZnO at ambient temperature in methanol dispersion for DMPO-O2•- ([DMPO]=0.20M, mcat=2.5 mg, Vsolvent=0.5 mL). | |
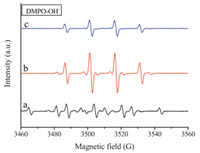
|
Download:
|
| Figure 8. DMPO spin-trapping ESR spectra recorded for (a) ZnTi-PABA-LDH, (b) TiO2 and (c) ZnO at ambient temperature in aqueous dispersion for DMPO-OH• ([DMPO]=0.20M, mcat=2.5 mg, Vsolvent=0.5 mL). | |
4. Conclusion
As a good organic UV absorbent, PABA has been successfully intercalated into ZnTi-LDH by an anion-exchange method. After intercalation of PABA, the basal spacing increases from 0.669 nm in the precursor to 0.913 nm for ZnTi-PABA-LDH. Analysis of FT-IR, SEM, TEM and UV-vis diffuse reflectance spectra indicates that there are complex supramolecular interactions between the host layers and guest anions in the intercalated structure. UV-vis diffuse reflectance spectra also show that the UV absorption of ZnTi-PABALDH is higher and broader than both the ZnTi-LDH precursor and pristine PABA, Furthermore, the ZnTi-LDH precursor exhibits stronger anti-ultraviolet ability. The UV absorbent-intercalated hybrid shows much better thermostability than the pristine UV absorbent. In addition, compared with the frequently employed ultraviolet blocking agents, TiO2 and ZnO, the ZnTi-PABA-LDH generate relatively lower active radicals (OH• and O2•-). These advantages enable ZnTi-PABA-LDH composites to have potential applications as sunscreen materials.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21301012).
| [1] | Andrady A.L., Hamid H., Torikai A.. Effects of solar UV and climate change on materials. Photochem. Photobiol. Sci. 10 (2011) 292–300. DOI:10.1039/c0pp90038a |
| [2] | Matsumura Y., Ananthaswamy H.N.. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 195 (2004) 298–308. DOI:10.1016/j.taap.2003.08.019 |
| [3] |
(a) W. Stahl, H. Sies, Carotenoids and protection against solar UV radiation, Skin Pharmacol. Appl. Skin Physiol. 15(2002) 291-296; (b) F. Xing, B. Zhao, W. Shi, Study on Tunable Fabrication of the Ultra-black Ni-P Film and its Blacking Mechanism, Electrochim. Acta 100(2013) 157-163. |
| [4] | Wong T., Orton D.. Sunscreen allergy and its investigation. Clin. Dermatol. 29 (2011) 306–310. DOI:10.1016/j.clindermatol.2010.11.002 |
| [5] | Zhao Y.B., Lin H.Y., Chen M.X., Yan D.P.. Niflumic anion intercalated layered double hydroxides with mechano-induced and solvent-responsive luminescence. Ind. Eng. Chem. Res. 53 (2014) 3140–3147. DOI:10.1021/ie404054v |
| [6] | Wang G.R., Xu S.M., Xia C.H., et al. Fabrication of host-guest UV-blocking materials by intercalation of fluorescent anions into layered double hydroxides. RSC Adv. 5 (2015) 23708–23714. DOI:10.1039/C5RA00589B |
| [7] | Sideris P.J., Nislsen U.G., Gan Z.H., Grey C.P.. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 321 (2008) 113–117. DOI:10.1126/science.1157581 |
| [8] | Li L., Ma R.Z., Ebina Y., et al. Layer-by-layer assembly and spontaneous flocculation of oppositely charged oxide and hydroxide nanosheets into inorganic sandwich layered materials. J. Am. Chem. Soc. 129 (2007) 8000–8007. DOI:10.1021/ja0719172 |
| [9] | Yan D.P., Wei M.. Photofunctional Layered Materials, Springer International Publishing. Switzerland (2015) . |
| [10] | Fogg A.M., Green V.M., Harvey H.G., O'Hare D.. New separation science using shape-selective ion exchange intercalation chemistry. Adv. Mater. 11 (1999) 1466–1469. DOI:10.1002/(ISSN)1521-4095 |
| [11] | Choy J.H., Kwak S.Y., Park J.S., Jeong Y.J., Portier J.. Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J. Am. Chem. Soc. 121 (1999) 1399–1400. DOI:10.1021/ja981823f |
| [12] | Wang M.Z., Li Y., Ji J.J., et al. Novel hybrids of Cu2+ ternary complexes of salicylidene-amino acid Schiff base with phenanthroline (or bipyridine) intercalated in Mg/Al-NO3-layered double hydroxide. Chin. Chem. Lett. 24 (2013) 593–596. DOI:10.1016/j.cclet.2013.03.040 |
| [13] | Zhao L., Zhou J.J., Huang X.Y., et al. Design, synthesis and anti-proliferative effects in tumor cells of new combretastatin A-4 analogs. Chin. Chem. Lett. 26 (2015) 993–999. DOI:10.1016/j.cclet.2015.05.003 |
| [14] | Wang X.R., Li Y., Tang L.P., et al. A reversible photochromic switch based on selfassembly of layered double hydroxide and decatungstate. Sens. Actuators B 223 (2016) 634–640. DOI:10.1016/j.snb.2015.09.147 |
| [15] | Das N., Samal A.. Synthesis, characterisation and rehydration behaviour of titanium(IV) containing hydrotalcite like compounds. Microporous Mesoporous Mater. 72 (2004) 219–225. DOI:10.1016/j.micromeso.2004.04.004 |
| [16] | J. He, M. Wei, B. Li, et al., Preparation of layered double hydroxides, in:X. Duan, D.G. Evans (Eds.), Layered Double Hydroxides, vol. 119, Springer, Berlin/Heidelberg, 2006, pp. 89-119. |
| [17] | Abdolmohammad-Zadeh H., Falaghi S., Rahimpour E.. An innovative nano-sorbent for selective solid-phase extraction and spectrophotometric determination of p-amino benzoic acid in cosmetic products. Int. J. Cosmetic Sci. 36 (2014) 140–147. DOI:10.1111/ics.2014.36.issue-2 |
| [18] | Cantrell T., Jussel N., Morris S.. Sun damage and skin protection. Pharmaceutics 425 (2002) 1–8. |
| [19] | Darvay A., White I.R., Rycroft R.J.G., et al. Photoallergic contact dermatitis is uncommon. Br. J. Dermatol. 145 (2001) 597–601. DOI:10.1046/j.1365-2133.2001.04458.x |
| [20] | Perioli L., Ambrogi V., Bertini B., et al. Anionic clays for sunscreen agent safe use:photoprotection, photostability and prevention of their skin penetration. Eur. J. Pharm. Biopharm. 62 (2006) 185–193. DOI:10.1016/j.ejpb.2005.08.001 |
| [21] | Saber O., Tagaya H.. New layered double hydroxide, Zn-Ti LDH:preparation and intercalation reactions. J. Incl. Phenom. Macrocyclic. Chem. 45 (2003) 107–115. DOI:10.1023/A:1023078728942 |
| [22] | Kloprogge J.T., Wharton D., Hickey L., Frost R.L.. Infrared and Raman study of interlayer anions CO32-, NO3-, SO42-, ClO4- in Mg/Al-hydrotalcite. Am. Mineral. 87 (2002) 623–629. DOI:10.2138/am-2002-5-604 |
| [23] | Ma H.Y., Gao R., Yan D.P., Zhao J.W., Wei M.. Organic-inorganic hybrid fluorescent ultrathin films and their sensor application for nitroaromatic explosives. J. Mater. Chem. C 1 (2013) 4128–4137. DOI:10.1039/c3tc30142g |
| [24] | Gao R., Zhao M.J., Guan Y., et al. Ordered and flexible lanthanide complex thin films showing up-conversion and color-tunable luminescence. J. Mater. Chem. C 2 (2014) 9579–9586. DOI:10.1039/C4TC01213E |
| [25] | Yamamoto Y., Imai N., Mashima R., et al. Singlet oxygen from irradiated titanium dioxide and zinc oxide. Methods Enzymol. 319 (2000) 29–37. DOI:10.1016/S0076-6879(00)19005-6 |
| [26] | Corazzari I., Livraghi S., Ferrero S., et al. Inactivation of TiO2 nano-powders for the preparation of photo-stable sunscreens via carbon-based surface modification. J. Mater. Chem. 22 (2012) 19105–19112. DOI:10.1039/c2jm32876c |
 2016, Vol. 27
2016, Vol. 27 


