b Shanghai Electrochemical Energy Devices Research Center, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
The rechargeable non-aqueous lithium-oxygen (Li-O2) batteries are recently attracting increasing research attention due to their higher specific energy density compared with conventional lithium-ion batteries (LIBs). It is expected that Li-O2 batteries could be widely applied as new energy resources in electric vehicles (EVs) and other electric devices [1, 2]. Despite much research efforts have been devoted on Li-O2 batteries, some significant barriers still limit the practical application of Li-O2 batteries. One of the main challenges is to develop a stable electrolyte for Li-O2 batteries. Initial works on the electrolytes for Li-O2 batteries were focused on organic carbonates such as propylene carbonate (PC), ethylene carbonate (EC) and dimethyl carbonate (DMC) based electrolytes due to their high boiling points and high ionic conductivities [3, 4]. But those carbonates were later found to be unstable in the presence of superoxide radical (O2-), an intermediate phase formed from oxygen reduction reaction during discharge, and easily reacted with Li2O2 to form unwanted products like Li2CO3 or LiOH [3, 4]. Later, ionic liquids (ILs) based electrolytes were used in Li-O2 batteries due to their relatively good electrochemical and chemical stability against nucleophilic attack of the superoxide radical (O2-) [4-6]. However, ILs still possess several shortcomings: high voscosity, low lithium salt solubility, low ionic conductivity and moisture susceptibility, which prevent their practical use in Li-O2 batteries [4]. Furthermore, the recent researches paid attention to ethers such as dimethoxy ethane (DME) [7], 1, 3-dioxolane [7] and tetraethylene glycol dimethyl ether (TEGDME) as electrolytes [8]. Unfortunately, all of them were not stable in the ambient of Li2O2. Another general electrolyte used in Li-O2 battery was dimethyl sulfoxide (DMSO) based electrolytes [9], which showed good cycling stability and high capacity with normal carbon air-electrodes in Li-O2 batteries. However, DMSO is not stable with bare metallic lithium, then it is required to a protected treatment for the metallic lithium electrode before used. In addition, DMSO is volatility, and deep research found that the formation of Li2O2 was still accompanied by unwanted by-products when carbon materials were employed as the air-electrodes in Li-O2 batteries [10].
Herein, we investigate ethylene sulfite (ES) as a possible electrolyte solvent for Li-O2 batteries. ES has been suggested previously as electrolyte solvent for lithium batteries, mostly in combination with metallic lithium [11, 12]. It was reported that ES was stable with bare metallic lithium [11, 12]. Compared to DMSO, ES has also superior properties including much lower volatility and higher electrochemical stability (see Table 1). In addition, the main reason of the decomposition of DMSO and other organic carbonates solvents is that the α-CH hydrogen atoms are acidic and vulnerable to the nucleophilic attack from superoxide radical (O2-) [13]. ES will not be easily attacked by the superoxide radical (O2-) because there are no a-CH hydrogen atoms in its structure (see Table 1). Then we expect that ES based electrolyte will be more stable in Li-O2 batteries. For these reasons, we employed ES as a novel electrolyte solvent and evaluated its performance in Li-O2 batteries. To the best of our knowledge, it is the first time to report that ES based electrolyte was used in Li-O2 batteries.
|
|
Table 1 Comparison of physical and chemical properties of DMSO and ES. |
2. Experimental 2.1. Materials
Battery-grade ethylene sulfite (ES) and lithium bis(trifluoromethanesulfonyl) imide salt (LiTFSI) were purchased from SigmaAldrich, and used without further purification. Multi-wall carbon nanotubes (MWCNTs) (range of diameter: 10-30 nm, length: >2 mm, special surface area: 160-200 m2 g-1) were purchased from the Shenzhen Nanotech Port Co., Ltd., China. The electrolyte of 1 mol L-1 LiTFSI in ES was prepared in an argon-filled glove box, in which both oxygen and water contents were less than 1 ppm.
2.2. Electrochemical measurementsThe air-electrodes for the Li-O2 batteries were prepared by mixing MWCNTs (as electrode material) and poly(vinylidene fluoride) (PVDF as binder) in 1-methyl-2-pyrrolidone (NMP) solvent, then casting onto a carbon paper current collector, and followed vacuum-drying at 80 ℃ for 24 h. The weight ratio of MWCNTs:PVDF (after drying) in the air-electrodes was 8:2. The loading of MWCNTs electrode material was 0.3 mg cm-2 in our experiments.
A modified two electrode CR2032 coin-cell configuration with six evenly dispersed holes at the positive case (Fig. 1) for electrochemical test was assembled with the prepared airelectrodes as cathode, metallic lithium foil (1.56 cm diameter and 0.45 mm thick) as anode, and 1 mol L-1 LiTFSI in ES as electrolyte. A Whatman glass filter paper (~28 mm diameter) was used as separator in the Li-O2 batteries. The assembling process was carried out in an argon-filled glove box with less than 0.1 ppm of both oxygen and moisture. The assembled Li-O2 batteries were placed in a gastight vessel continuously saturated with 1.0 atm of high-purity oxygen (99.999%), and tested on Land CT2001A test system (Wuhan, China) at 60 ℃.

|
Download:
|
| Figure 1. Photographic image of the lithium–oxygen battery used in this work. | |
2.3. Characterization
Electrochemical window of the ES based electrolyte was examined from 0 V to 6 V at 10 mV s-1 under pure Ar atmosphere on an Autolab PGSTAT302 electrochemical test system (Eco Chemie, the Netherlands). In our experiments, lithium foil was used as both counter and reference electrodes, stainless steel was used as working electrode, and 1 mol L-1 LiTFSI in ES was used as electrolyte, respectively. X-ray diffraction (XRD) measurements were carried out on an X-ray diffractometer (D/max-2200/PC, Japan) with Cu-Ka radiation from 10° to 80° at a scanning rate of 0.1° s-1. For XRD measurements of the cycled air-electrodes, the air-electrodes were extracted from the Li-O2 batteries immediately after discharge in an argon-filled glove-box and sealed in an airtight XRD sample holder with a dome that screwed on with a rubber O-ring fitting before being taken to the XRD to minimize air exposure.
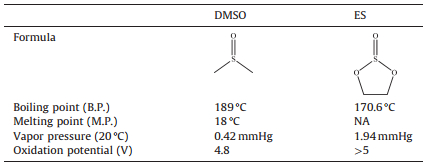
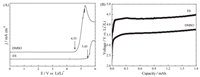
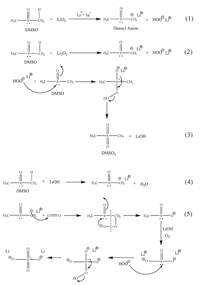
3. Results and discussionNow, DMSO is one of the normally used solvents in Li-O2 batteries. However, DMSO has high volatility, which will limit it to be used in Li-air batteries operated in ambient air environments. From Table 1, it can be found that ES has a higher boiling point, and higher vapor pressure value at 20 ℃ compared with DMSO. This means that ES has lower volatility than that of DMSO. The electrochemical window of ES and DMSO were determined at room temperature under Ar atmosphere. As shown in Fig. 2A, it can be found that the oxidation potentials were found to be 4.55 V and 5.45 V versus Li/Li+ for DMSO and ES solvents, respectively. This indicates that ES has good resistance toward electro-oxidation. In Li-O2 battery system, it generally requires that an electrolyte should endure high over-potential during charge and thus superior antioxidant ability. Fig. 2B shows the decomposition voltage of both DMSO and ES solvents in Li-O2 batteries under O2 atmosphere during charge without the previous discharge processes. It can be found that the stability of ES was higher than that of DMSO, and there were no any decomposition until 4.50 V for ES solvent. These results indicate that ES solvent has good electrochemical stability under O2 atmosphere, which will provide a stable electrolyte for Li-O2 batteries. Fig. 3 shows the degradation mechanism of DMSO used in lithium oxygen battery [14]. The methyls of DMSO are weakly acidic and can serve as a proton source with strong bases, generating the resonance stabilized dimsyl(methylsulfinyl) anion. Furthermore, the sulfoxide linkage of DMSO can react with oxidizing agents to yield the corresponding sulfone (DMSO2). The degradation mechanism of DMSO is clearly depicted in Fig. 3. Obviously, when using carbon cathode, there are the formation of side products resulting from exposure of DMSO to the O2-and O2-2, aggressive nucleophiles and bases, formed during the ORR. In contrast, there is no -CH3 in the structure of ES, thus, ES is not easily attacked by the superoxide radicals like O2-to form the unwanted by-products such as LiOH, lithium sulfite (Li2SO3) and ultimately lithium sulfate (Li2SO4). Then, the stability of ES will be higher than that of DMSO in a Li-O2 battery.
|
Download:
|
| Figure 2. (A) Electrochemical windows of DMSO and ES solvents. (B) Decomposition voltage of DMSO and ES solvents in a Li–O2 battery under O2 atmosphere. | |
|
Download:
|
| Figure 3. Degradation mechanism of DMSO used in lithium oxygen battery. | |
A Li-O2 battery with 1 mol L-1 LiTFSI in ES electrolyte was investigated at 60 ℃ in our experiments. The air-electrode was made of MWCNTs and PVDF binder without any catalysts. Fig. 4A shows the first charge and discharge curves of the Li-O2 battery at a current density of 200 mA g-1. It can be found that the discharge and charge voltage plateaus are 2.7 V and 4.26 V, respectively. The specific discharge and charge capacities are 4929 mAh g-1 and 3949 mAh g-1 respectively, corresponding to a high round-trip efficiency of 80.1%. The performance obtained in the Li-O2 battery is much higher than that of the most advanced LIBs, and is also similar to the Li-O2 battery using the DMSO based electrolyte and carbon based air-electrode (without catalysts used) [15]. Fig. 4B shows the XRD patterns of the air-electrodes at different discharge-charge stages in the Li-O2 batteries using 1 mol L-1 LiTFSI in ES electrolyte. It can be found that the main discharge product is Li2O2. After charging, all the peaks of Li2O2 were disappeared, which indicates the discharge product Li2O2 was completely decomposed during the charging process. These results show that the Li-O2 battery with ES based electrolyte has a good reversibility.

|
Download:
|
| Figure 4. (A) First discharge–charge voltage profiles of Li–O2 battery using 1 mol L-1 LiTFSI in ES electrolyte at a current density of 200 mA g-1. (B) XRD spectra of the airelectrode at different charge–discharg charged states. | |
Cycling stability of the Li-O2 battery with 1 mol L-1 LiTFSI in ES electrolyte was tested by the recently widely used capacity-limited cycle method [16]. Fig. 5A and B shows the cycling stability of the Li-O2 battery cycled at a fixed capacity of 500 mAh g-1 and a current density of 200 mA g-1. It can be found that the Li-O2 battery with 1 mol L-1 LiTFSI in ES electrolyte can keep 8 cycles, and the discharge voltage remains up to 2.5 V. We extended the battery test by determining the cycling response under high specific capacity limit (specific capacity: 1000 mAh g-1 shown in Fig. 5c). It can also be found that the Li-O2 battery with 1 mol L-1 LiTFSI in ES electrolyte shows good cycling stability. We also test the Li-O2 battery with 1 mol L-1 LiTFSI in DMSO electrolyte under the same conditions. However, the battery using DMSO based electrolyte showed poor cycling stability, and cannot be cycled for 2nd cycle. Maybe the main reason is the unstable property in the presence of superoxide radicals and the high volatility of DMSO, especially at high temperature. In addition, we also found the Li-O2 battery exhibited slight performance decay, as indicated by an increasing voltage gap between charge and discharge voltages upon increasing cycles. In general, there are several main factors resulting in the cycling performance decay of Li-O2 batteries [3, 4]: (1) The by-products from the parasitic reactions of MWCNTs and PVDF with the superoxide radical (O2-) blocked the porous of airelectrodes, (2) degradation of Li metal caused by dendrite growth, and (3) decomposition of electrolyte. From the literature, the main reason of the decomposition of DMSO and other organic carbonates solvents is that the α-CH hydrogen atoms are acidic and vulnerable to the nucleophilic attack from superoxide radical (O2-) [13].

|
Download:
|
| Figure 5. (A) Discharge–charge voltage profiles and (B) variation of voltage on the terminal of discharge and charge of Li–O2 battery cycled at a fixed capacity of 500 mAh g-1. (C) Variation of voltage on the terminal of discharge and charge of Li–O2 battery cycled at a fixed capacity of 1000 mAh g-1. Current density: 200 mA g-1. | |
However, there are no α-CH hydrogen atoms in ES, which will not be easily attacked by the superoxide radical (O2-). Then we think that the ES based electrolyte will be more stable in Li-O2 batteries. We believe that the performance of Li-O2 battery can be further improved by employing stable air-electrode catalysts and binder materials, optimization of air-electrode structures with extended reaction zones.
4. ConclusionIn summary, we firstly report and demonstrate that a novel ES based electrolyte can be used in non-aqueous Li-O2 batteries. Due to no α-CH hydrogen atoms in ES, we think that ES solvent will not be easily attacked by the superoxide radical (O2-). Then, good electrochemcial performance in Li-O2 batteries including high specific capacity, good round-trip efficiency and cycling stability was successfully obtained. The main discharge product was confirmed to be the desired Li2O2. Our initial feasibility study shows that ES based electrolyte will provide a promising strategy to develop Li-O2 batteries with high performance.
AcknowledgmentsThe work was supported by the National Key Basic Research Program of China (No. 2014CB932303), the National Natural Science Foundation of China (No. 21573145).
| [1] | Bruce P.G., Freunberger S.A., Hardwick L.J., Tarascon J.-M.. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11 (2012) 19–29. |
| [2] | Scrosati B., Hassoun J., Sun Y.-K.. Lithium-ion batteries. a look into the future. Energy Environ. Sci. 4 (2011) 3287–3295. DOI:10.1039/c1ee01388b |
| [3] | Lu J., Li L., Park J.-B., et al. Aprotic and aqueous Li-O2 batteries. Chem. Rev. 114 (2014) 5611–5640. DOI:10.1021/cr400573b |
| [4] | Balaish M., Kraytsberg A., Ein-Eli Y.. A critical review on lithium-air battery electrolytes. Phys. Chem. Chem. Phys. 16 (2014) 2801–2822. DOI:10.1039/c3cp54165g |
| [5] | Ferrari S., Quartarone E., Tomasi C., et al. Investigation of ether-based ionic liquid electrolytes for lithium-O2 batteries. J. Electrochem. Soc. 162 (2015) A3001–A3006. DOI:10.1149/2.0101511jes |
| [6] | Das S., Højberg J., Knudsen K.B., et al. Instability of ionic liquid-based electrolytes in Li-O2 batteries. J. Phys. Chem. C 119 (2015) 18084–18090. DOI:10.1021/acs.jpcc.5b04950 |
| [7] | Zhao G.Y., Zhang L., Wang B.Y., Sun K.N.. Cuprous oxide as cathode catalysts of lithium oxygen batteries. Electrochim. Acta 184 (2015) 117–123. DOI:10.1016/j.electacta.2015.10.059 |
| [8] | Jung H.G., Hassoun J., Park J.-B., Sun Y.-K., Scrosati B.. An improved high-performance lithium-air battery. Nat. Chem. 4 (2012) 579–585. DOI:10.1038/nchem.1376 |
| [9] | Peng Z.Q., Freunberger S.A., Chen Y.H., Bruce P.G.. A reversible and higher-rate Li-O2 battery. Science 337 (2012) 563–566. DOI:10.1126/science.1223985 |
| [10] | David G.K., Thomas P.B., Chibueze V.A., et al. Chemical instability of dimethyl sulfoxide in lithium-air batteries. J. Phys. Chem. Lett. 5 (2014) 2850–2856. DOI:10.1021/jz5013824 |
| [11] | Wrodnigg G.H., Besenhard J.O., Winter M.. Ethylene sulfite as electrolyte additive for lithium-ion cells with graphitic anodes. J. Electrochem. Soc. 146 (1999) 470–472. DOI:10.1149/1.1391630 |
| [12] | T. Yamahira, Y. Imamura, Japan Patent 08-096851, 1996. |
| [13] | Amanchukwu C.V., Harding J.R., Yang S.-H., Hammond P.T.. Understanding the chemical stability of polymers for lithium-air batteries. Chem. Mater. 27 (2015) 550–561. DOI:10.1021/cm5040003 |
| [14] | Sharon D., Afri M., Noked M., et al. Oxidation of dimethyl sulfoxide solutions by electrochemical reduction of oxygen. J. Phys. Chem. Lett. 4 (2013) 3115–3119. DOI:10.1021/jz4017188 |
| [15] | Xu D., Wang Z.L., Xu J.J., et al. A stable sulfonebasedelectrolyteforhigh performance rechargeable Li-O2 batteries. Chem. Commun. 48 (2012) 11674–11676. DOI:10.1039/c2cc36815c |
| [16] | Xu J.J., Wang Z.L., Xu D., Zhang L.L., Zhang X.B.. Tailoring deposition and morphology of discharge products towards high-rate and long-life lithium-oxygen batteries. Nat. Commun. 4 (2013) 2438. |
 2016, Vol. 27
2016, Vol. 27