The growing use of fossil fuels has highly promoted the development of industrial revolution, but it also caused rapid increase of CO2 emissions in the atmosphere and led to serious global climate warming. Since the past decade, scientists over the world have devoted great efforts to investigating series of porous materials for CO2 capture and storage (CCS) [1-3]. As an important family of porous materials, porous organic frameworks (POFs) which usually constructed from wholly light elements (C, H, O, B, etc.) had attracted scientists wide attention and became one of alternative materials for CCS, because of their large surface areas and good mechanical properties. Most POF materials, including polymers of intrinsic microporosity (PIMs) [4, 5], conjugated microporous polymers (CMPs) [6, 7], hyper-crosslinked polymers (HCPs) [8], triazine-based organic frameworks (CTFs) [9, 10], and porous aromatic frameworks (PAFs) [11-13] have been used for CCS [14, 15]. Besides, the variety of synthetic routes and ease of controlling pore properties in skeletons make POF materials been utilized in many fields, such as gas storage [16], separation [17], heterogeneous catalysis [18, 19], sensors [20] and electricity [21]. However, most of these materials are synthetized via noble metals involved reactions such as palladium catalyzed Sonogashira-Hagihara cross-coupling reaction [22], palladium catalyzed Suzuki cross-coupling reaction [23] and nickel catalyzed Yamamoto-type Ullmann cross-coupling reaction [24] or under high temperatures [9, 10]. These catalysts are very expensive and usually oxygen-sensitive which limited the large-scale development of the resulting POF materials. Recently, Cooper and Tan group reported the preparation of a series of HCPs with small heterocyclic molecular as monomers (pyrrole, thiofuran, furan, benzene and biphenyl, etc.) by Friedel-Crafts alkylation reaction [25-27]. These HCPs materials exhibited certain CO2 sorption abilities. Yuan and Jiang group have prepared a mass of polymers used planar monomers (1, 3, 5-triphenylbenzene, cyanuric chloride, tetraphenylethylene and 1, 1, 2, 2-tetraphenylethane-1, 2-diol, etc.) as building units [28-30]. Cubic units (such as octavinylsilsesquioxane) and tetrahedral molecules were also used to synthesize POF materials via Friedel-Crafts alkylation reaction [31]. This kind of reaction is catalysed by low-cost anhydrous FeCl3 or AlCl3. In the presence of Lewis acid, aromatic nuclei of monomers become active to achieve polymerization to product POF materials. Meanwhile, these materials polymerized by Friedel-Crafts alkylation reaction have many applications in electricity chemistry [32] and gas separation [33]. From the above, it suggested that Friedel-Crafts alkylation reaction could be an ideal candidate reaction for preparing cost-effective POF materials.
It is well known that diamond is one of the most stable compounds in nature. Tetrahedral organic molecules as components have many applications in design and preparation of supramolecular architectures and covalent polymers [34]. As a representable POF material with diamond-like structure, PAF-1 built from tetraphenylmethane (TPM) has ultra-high BET surface area (5600 m2 g-1) and good thermal and chemical stability [11]. Then our group promoted to synthesize three PAFs with TPM units and successfully incorporated functional groups (hydroxyl and amino group) in the skeleton with the alkylation reaction [35]. After that, Marchese group reported the synthesis of mPAF based on TPM building unit and carried out CO2 adsorption simulation with theoretical modeling, which confirmed that the POFs derived from tetrahedral monomers were expected to perform well in the gas uptake, especially at low pressures [36].
Herein, we presented the synthesis of a novel PAF material, PAF-8, choosing diamond-like structural monomer tetraphenylsilane, and using Friedel-Crafts alkylation reaction catalysed by anhydrous FeCl3 under mild condition. Adequate characterizations have been employed to confirm the structural features and pore properties of the resulting PAF material, and it turned out that PAF-8 was synthesized as desired. Beside from the good stability and high surface area (785 m2 g-1), PAF-8 also presented good performance on gas and liquid sorption such as CO2, CH4, water and methanol. In addition, gas chromatography experiment was carried out based on the resulting data and PAF-8 material exhibited good separation abilities for CH4-CO2 and N2-CO2 mixture.
2. Experimental 2.1. ChemicalsTetraphenylsilane and formaldehyde dimethyl acetal (FDA) were purchased from Alfa Aesar. CaH2 was purchased from Aladdin. Other chemicals and reagents were purchased from commercial suppliers without further purification unless otherwise stated. Methylene dichloride (CH2Cl2) was dehydrated with CaH2.
2.2. Synthesis of PAF-8Tetraphenylsilane (1 mmol, 0.336 g) and FeCl3 (4 mmol, 0.65 g) were added into a 50 mL round-bottom-flask and then this system was purged with Ar by three freeze-pump-thaw cycles. FDA (4 mmol 0.36 mL) and CH2Cl2 (10 mL) were injected into the flask with a syringe. The polymerization fused aromatic monomers with 4 equivalents of FDA (depending on the number of aromatic reaction sites available). This mixture was stirred at 45 ℃ for 24 h to produce the PAF material. After cooling down to room temperature, the resulting powders were collected by filtration and washed with 1 mol L-1 hydrochloric acid solution, acetone and methanol until the filtrate liquid was nearly colorless. Then the product was Soxhlet extracted for 24 h with methanol, tetrahydrofuran and dichloromethane, respectively to remove the monomers and catalysts thoroughly and then dried under vacuum to give PAF-8 as brown powders (96% yield, Fig. 1).

|
Download:
|
| Figure 1. Synthetic pathway of PAF-8. | |
2.3. Physical measurements
The Fourier transform infrared spectroscopy (FTIR) spectra (film) were measured using IFS 66V/S Fourier transform infrared spectroscopy. Solid-state 13C CP/MAS NMR measurement was performed on a BrukerAvance Ⅲ model 400 MHz spectrometer at a MAS rate of 5 kHz. The Element analysis (for C and H) were measured using a Perkin Elmer 2400 Series CHNS/O Analyzer. The powder X-ray diffraction (PXRD) was performed by a Riguku D/ MAX 2550 diffractometer using Cu-Kα radiation, 50 kV, 200 mA with scanning rate of 4° min-1 (2θ). Scanning electron microscopy (SEM) analysis was performed on a JEOS JSM 6700. Transmission electron microscopy (TEM) was recorded using a JEOL JEM 3010 with an acceleration voltage of 300 kV. Thermogravimetric analysis (TGA) was performed using a Netzch Sta449c thermal analyzer system at a heating rate of 10 ℃ min-1 in air. The gas adsorption-desorption isotherms were measured on a Quantachrome Autosorb-iQ2 analyzer. Gas chromatography (GC) separation of selected light gases using a column packed with PAFs was performed with a SP-2100A GC system equipped with a thermal conductivity detector.
3. Results and discussionThe structure properties of PAF-8 were characterized by various spectroscopic measurements. FTIR spectra, 13C CP/MAS NMR spectrum and elemental analysis were performed to confirm the structural features and the polymerization degree. PXRD, SEM and TEM were carried out to discover the morphology and crystallinity. The pore character was studied by N2 sorption isotherm measurement. Because of the permanent porosity and high surface area of PAF-8, CO2 and CH4 adsorption and separation were also carried out. And the results indicated this PAF material had good gas sorption and separation abilities. Additionally, PAF-8 exhibited high water and methanol sorption abilities.
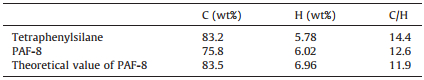
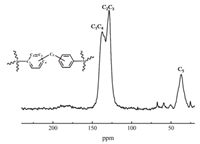
Comparisons of the FTIR spectra of PAF-8 and its tetrahedral monomer were performed to confirm the structural features and reaction degree of polymerization. As shown in Fig. 2, the appearance of intense saturated C-H bands (at 2903 cm-1) of PAF-8 demonstrated the networks were linked by methylene group as desired. The peaks at ca. 3000 cm-1 belong to aromatic C-H stretching vibrations and the peaks at ca. 1500 cm-1 belong to aromatic C-H bending vibrations. The reduction in peak intensity of aromatic C-H bending vibrations was affected by the introduction of methylene group which indicated that reaction degree of polymerization was complete relatively. The aromatic C=C stretching vibrations at 1630 cm-1 indicated the existence of phenyl rings in the PAF-8 network. These results displayed the success of Friedel-Crafts alkylation reaction and the integrity of the aromatic skeleton. The local structure of PAF-8 was further investigated by 13C CP/MAS NMR spectrum. In the 13C CP/MAS NMR spectrum of PAF-8 (Fig. 3), three different types of resonance peaks were clearly observed. The strong signals in the range of 140 ppm-125 ppm were attributed to the aromatic carbon atoms. The peak at 127 ppm belonged to the aromatic C (C2, C3) connected with H and the peak at 138 ppm belonged to the aromatic C (C1, C4) connected with other atom or perssad. Moreover, the signal near 37 ppm was related to the methylene carbon atoms (C5), thus further indicating the FDA participated in the Friedel-Crafts alkylation reaction. From the results of FTIR and 13C CP/MAS NMR, PAF-8 was synthetized successfully as expected. Elemental analysis (for C and H. Table 1) were measured to determine their content quantitatively and the result confirmed the methylene group participated in the alkylation. Because of the introduction of methylene group, the C/H ratio decreased slightly. It was consistence with the result of elemental analysis measurement. All of these showed that success of Friedel-Crafts alkylation reaction and the networks were linked by methylene group as desired.

|
Download:
|
| Figure 2. FTIR spectra of PAF-8 (top) and tetraphenylsilane (bottom). | |
|
Download:
|
| Figure 3. 13C CP/MAS NMR spectrum of PAF-8. | |
|
|
Table 1 13C CP/MAS NMR spectrum of PAF-8. |
In order to confirm the long-range structure of PAF-8, PXRD was carried out to discover the crystallinity. There were no intense peaks but only a broad peak indicating that the texture of the polymer was amorphous which might be caused by the irreversibility of Friedel-Crafts alkylation reaction and introducing of flexible perssad (methylene). This is common in most POF materials which synthesized via kinetics-controlled irreversible coupling processes [22, 35]. SEM image (Fig. 4a) showed that PAF-8 was amorphous and most particles were micro-sized irregular blocks. And its dimensions ranged from 200 nm to 800 nm. From the TEM (Fig. 4b), no ordered and obvious pore structure could be discovered which indicated the arrangement of the pores was random. This result was consistent with PXRD.

|
Download:
|
| Figure 4. SEM (a) and TEM (b) images of PAF-8. | |
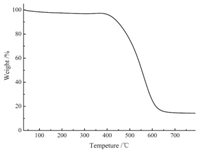
TGA under air condition was employed to investigate the structure stability of the polymers (Fig. 5). PAF-8 displayed high thermal stability with decomposition temperature above 350 ℃. Because of the existence of Si element, there was 16% oxide residue. It was consistent with the Si element content in PAF-8. In addition, PAF-8 material cannot be dissolved and decomposed in common organic solvents and acid solution, such as DMF, THF, CHCl3, 1 mol L-1 HCl acid solution and so on, which indicated its high chemical stability.
|
Download:
|
| Figure 5. TGA plot of PAF-8. | |
The pore character of PAF-8 was studied by N2 sorption isotherm measured at 77 K under 1 atm. As shown in Fig. 6, there was a sharp uptake at low relative pressures, indicating PAF-8 possessed microporous texture. A distinct hysteresis loop was also clearly observed in the desorption branch, which is a feature of classic type IV isotherm, suggesting the presence of mesopores. The apparent surface area of PAF-8 calculated from the Brunauer-Emmett-Teller (BET) model is 785 m2 g-1. The pore size distribution was calculated from nonlocal density functional theory (NLDFT). It gave two narrow peaks at 1.69 nm and 3.80 nm agreed with the N2 sorption isotherm. The high surface area and permanent porosity make PAF-8 an excellent candidate for gas sorption.

|
Download:
|
| Figure 6. N2 sorption isotherms and pore width distribution (inset) of PAF-8. | |
Gas sorption measurements were carried out because of the high surface area and permanent porosity of PAF-8. Industrial production CO2 and CH4 sorption were studied in our paper. CO2 and CH4 adsorption capacities of PAF-8 were measured at 273 K and 298 K, respectively. In Fig. 7a, CO2 adsorption quantity of PAF-8 at 273 K and 298 K are 35.5 cm3 g-1 and 19.4 cm3 g-1, respectively. The heat of adsorption (Qst) of PAF-8 was calculated from adsorption data collected from 273 and 298 K, using the Clausius-Clapeyron equation and the Qst of CO2 is 34.5 kJ mol-1. Its Qst of CO2 is higher than many other POF materials such as HCP materials (20-24 kJ mol-1) [37], polycarbazole CPOP-1 (27 kJ mol-1) [38], polybenzimidazole BILP-1 (26.5 kJ mol-1) [39]. The CH4 adsorption quantity of PAF-8 at 273 K and 298 K are 10.7 cm3 g-1 and 5.8 cm3 g-1 (Fig. 7c). The Qst of CH4 is 24.5 kJ mol-1, which is lower than Qst of CO2, thus indicating PAF-8 material possessed stronger sorption ability for CO2 compared with CH4. Due to the comparative CO2 and CH4 sorption capacity and different sorption ability, the separation measurement was also performed. To validate the sieving effect of PAF-8, the gas-chromatographic (GC) separation was employed at room temperature. PAF-8 was grinded and then packed tightly into the column (30 cm long×2 mm internal diameter). The lower end of the column was filled with glass wool during the column packing. GC with a SP-2100A GC system equipped with thermal conductivity detector was used to characterize the kinetic separation of CO2-CH4 mixture. The collected separation curve was shown in Fig. 7e. The result indicated that PAF-8 had very good separation ability of CO2 and CH4. Then, another nonpolar molecule N2 was chosen for the separation measurement. It happened that there was a similar case that CO2 and N2 mixture were also separated through PAF-8. The retention times of CH4, N2 and CO2 were 1.6, 2.6 and 12.5 min, respectively. The reason might be van der Waals interactions between skeleton and weak nonpolar CO2 were stronger than that between skeleton and strong nonpolar CH4 and N2, so CH4 and N2 flowed more quickly than CO2. The results suggested the promising application of PAF-8 in selective adsorption and separation of CO2.

|
Download:
|
| Figure 7. CO2 (a) and CH4 (c) sorption isotherms of PAF-8 and Qst of CO2 (b) and CH4 (d) measured at 273 K and 298 K, respectively, separation curves for N2–CO2 and CH4–CO2 mixtures of PAF-8 (e). | |
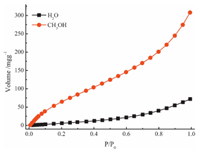
The inherent porosity of PAF-8 allows potential access by small solvent molecules, such as water and methanol. As shown in Fig. 8, PAF-8 material can adsorb certain amounts of water and methanol with value of 72 mg g-1 and 308 mg g-1 at 298 K. It indicated that PAF-8 material had high liquid vapor adsorption abilities. The adsorption amount of methanol is higher than water which showed that this PAF material may have potential application in H2O-CH3OH separation.
|
Download:
|
| Figure 8. Water and methanol vapor adsorption isotherms at 298 K. | |
4. Conclusion
In summary, the PAF material (PAF-8) based on tetrahedral building blocks was successfully synthesized by simple one-step Friedel-Crafts alkylation reaction in the presence of inexpensive catalyst FeCl3. The properties of PAF-8 material were well investigated and discussed. FTIR spectra and 13C CP/MAS NMR spectrum confirmed the structural features and the polymerization degree and the methylene group was introduced into the polymer skeletons successfully. TGA characterizations indicated that the PAF-8 material displayed high thermal stability. Meanwhile, PAF-8 material cannot be dissolved and decomposed in common organic solvents and acid solution. The apparent BET surface area of PAF-8 is 785 m2 g-1. It possessed both micropores and mesopores at 1.69 and 3.80 nm. PAF-8 also exhibited the great potential application in CO2 and CH4 capture and especially possessed high Qst of CO2 (34.5 KJ mol-1), which was higher than many other POF materials. N2-CO2 and CH4-CO2 mixture can also be separated through GC separation. Additionally, PAF-8 material had good water and methanol vapor sorption abilities. All of it indicated that PAF-8 material had good application foreground for CO2 capture and separation as well as for liquid vapor adsorption.
AcknowledgmentWe are grateful for the financial support of National Basic Research Program of China (973 Program, Nos. 2012CB821700 and 2014CB931804), Major International (Regional) Joint Research Project of NSFC (No. 21120102034) and NSFC Project (Nos. 21531003 and 21503038).
| [1] | Dawson R., Cooper A.I., Adams D.J.. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 62 (2013) 345–352. |
| [2] | Jiang J.X., Cooper A.I.. Microporous organic polymers:design, synthesis, and function. Top. Curr. Chem. 293 (2010) 1–33. |
| [3] | Zhu X.L., Wang P.Y., Peng C., Yang J., Yan X.B.. Activated carbon produced from paulownia sawdust for high-performance CO2 sorbents. Chin. Chem. Lett. 25 (2014) 929–932. DOI:10.1016/j.cclet.2014.03.039 |
| [4] | Budd P.M.. Putting order into polymer networks. Science 316 (2007) 210–211. DOI:10.1126/science.1141929 |
| [5] | Carta M., Croad M., Malpass-Evans R., et al. Triptycene induced enhancement of membrane gas selectivity for microporous Tröger's base polymers. Adv. Mater. 26 (2014) 3526–3531. DOI:10.1002/adma.v26.21 |
| [6] | Ding X.S., Han B.H.. Metallophthalocyanine-based conjugated microporous polymers as highly efficient photosensitizers for singlet oxygen generation. Angew. Chem. Int. Ed. 54 (2015) 6536–6539. DOI:10.1002/anie.201501732 |
| [7] | Du R., Zhang N., Xu H., et al. CMP aerogels:ultrahigh-surface-area carbon-based monolithic materials with superb sorption performance. Adv. Mater. 26 (2014) 8053–8058. DOI:10.1002/adma.v26.47 |
| [8] | Zhang J.S., Qiao Z.A., Mahurin S.M., et al. Hypercrosslinked phenolic polymers with well-developed mesoporous frameworks. Angew. Chem. Int. Ed. 54 (2015) 4582–4586. DOI:10.1002/anie.201500305 |
| [9] | Hao L., Zhang S.S., Liu R.J., et al. Bottom-up construction of triazine-based frameworks as metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 27 (2015) 3190–3195. DOI:10.1002/adma.201500863 |
| [10] | Zhu X., Tian C.C., Mahurin S.M., et al. A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation. J. Am. Chem. Soc. 134 (2012) 10478–10484. DOI:10.1021/ja304879c |
| [11] | Ben T., Ren H., Ma S.Q., et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 48 (2009) 9457–9460. DOI:10.1002/anie.200904637 |
| [12] | Wang W., Yuan Y., Sun F.X., Zhu G.S.. Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2. Chin. Chem. Lett. 25 (2014) 1407–1410. DOI:10.1016/j.cclet.2014.08.002 |
| [13] | Yuan Y., Sun F.X., Li L.N., Cui P., Zhu G.S.. Porous aromatic frameworks with aniontemplated pore apertures serving as polymeric sieves. Nat. Commun. 5 (2014) 4260. |
| [14] | Feng X., Ding X.S., Jiang D.L.. Covalent organic frameworks. Chem. Soc. Rev. 41 (2012) 6010–6022. DOI:10.1039/c2cs35157a |
| [15] | Kiskan B., Antonietti M., Weber J.. Teaching new tricks to an old indicator:pHswitchable, photoactive microporous polymer networks from phenolphthalein with tunable CO2 adsorption power. Macromolecules 45 (2012) 1356–1361. DOI:10.1021/ma202675v |
| [16] | Han S.S., Mendoza-Cortés J.L., Goddard Ⅲ W.A.. Recent advances on simulation and theory of hydrogen storage in metal-organic frameworks and covalent organic frameworks. Chem. Soc. Rev. 38 (2009) 1460–1476. DOI:10.1039/b802430h |
| [17] | Li J.R., Sculley J., Zhou H.C.. Metal-organic frameworks for separations. Chem. Rev. 112 (2012) 869–932. DOI:10.1021/cr200190s |
| [18] | Filer A., Choi H.J., Seo J.M., Baek J.B.. Two and three dimensional network polymers for electrocatalysis. Phys. Chem. Chem. Phys. 16 (2014) 11150–11161. DOI:10.1039/c4cp01246a |
| [19] | Zhang Y.G., Riduan S.N.. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 41 (2012) 2083–2094. DOI:10.1039/C1CS15227K |
| [20] | Xiang Z.H., Cao D.P.. Synthesis of luminescent covalent-organic polymers for detecting nitroaromatic explosives and small organic molecules. Macromol. Rapid Commun. 33 (2012) 1184–1190. DOI:10.1002/marc.201100865 |
| [21] | Feng X., Chen L., Honsho Y., et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor-acceptor ordering. Adv. Mater. 24 (2012) 3026–3031. DOI:10.1002/adma.v24.22 |
| [22] | Yuan R.R., Ren H., Yan Z.J., Wang A.F., Zhu G.S.. Robust tri(4-ethynylphenyl)aminebased porous aromatic frameworks for carbon dioxide capture. Polym. Chem. 5 (2014) 2266–2272. DOI:10.1039/c3py01252b |
| [23] | Weber J., Thomas A.. Toward stable interfaces in conjugated polymers:microporous poly(p-phenylene) and poly(phenyleneethynylene) based on a spirobifluorene building block. J. Am. Chem. Soc. 130 (2008) 6334–6335. DOI:10.1021/ja801691x |
| [24] | Schmidt J., Werner M., Thomas A.. Conjugated microporous polymer networks via Yamamoto polymerization. Macromolecules 42 (2009) 4426–4429. DOI:10.1021/ma9005473 |
| [25] | Luo Y.L., Li B.Y., Wang W., Wu K.B., Tan B.. Hypercrosslinked aromatic heterocyclic microporous polymers:a new class of highly selective CO2 capturing materials. Adv. Mater. 24 (2012) 5703–5707. DOI:10.1002/adma.v24.42 |
| [26] | Dawson R., Stevens L.A., Drage T.C., et al. Impact of water coadsorption for carbon dioxide capture in microporous polymer sorbents. J. Am. Chem. Soc. 134 (2012) 10741–10744. DOI:10.1021/ja301926h |
| [27] | Li B.Y., Gong R.N., Wang W., et al. A new strategy to microporous polymers:knitting rigid aromatic building blocks by external cross-linker. Macromolecules 44 (2011) 2410–2414. DOI:10.1021/ma200630s |
| [28] | Liu G.L., Wang Y.X., Shen C.J., Ju Z.F., Yuan D.Q.. A facile synthesis of microporous organic polymers for efficient gas storage and separation. J. Mater. Chem. A 3 (2015) 3051–3058. DOI:10.1039/C4TA05349D |
| [29] | Zhang Y.H., Li Y.D., Wang F., et al. Hypercrosslinked microporous organic polymer networks derived from silole-containing building blocks. Polymer 55 (2014) 5746–5750. DOI:10.1016/j.polymer.2014.09.014 |
| [30] | Yao S.W., Yang X., Yu M., Zhang Y.H., Jiang J.X.. High surface area hypercrosslinked microporous organic polymer networks based on tetraphenylethylene for CO2 capture. J. Mater. Chem. A 2 (2014) 8054–8059. DOI:10.1039/c4ta00375f |
| [31] | Yang W.Y., Wang D.X., Li L.G., Liu H.Z.. Construction of hybrid porous materials from cubic octavinylsilsesquioxane through Friedel-Crafts reaction using tetraphenylsilane as a concentrative crosslinker. Eur. J. Inorg. Chem. 2014 (2014) 2976–2982. DOI:10.1002/ejic.v2014.18 |
| [32] | Bildirir H., Osken I., Ozturk T., Thomas A.. Reversible doping of a dithienothiophene-based conjugated microporous polymer. Chem. Eur. J. 21 (2015) 9306–9311. DOI:10.1002/chem.v21.26 |
| [33] | Li H.Y., Meng B., Mahurin S.M., et al. Carbohydrate based hyper-crosslinked organic polymers with -OH functional groups for CO2 separation. J. Mater. Chem. A 3 (2015) 20913–20918. DOI:10.1039/C5TA03213J |
| [34] | Muller T., Bräse S.. Tetrahedral organic molecules as components in supramolecular architectures and in covalent assemblies, networks and polymers. RSC Adv. 4 (2014) 6886–6907. DOI:10.1039/c3ra46951d |
| [35] | Jing X.F., Zou D.L., Cui P., Ren H., Zhu G.S.. Facile synthesis of cost-effective porous aromatic materials with enhanced carbon dioxide uptake. J. Mater. Chem. A 1 (2013) 13926–13931. DOI:10.1039/c3ta13115g |
| [36] | Errahali M., Gatti G., Tei L., et al. Microporous hyper-cross-linked aromatic polymers designed for methane and carbon dioxide adsorption. J. Phys. Chem. C 118 (2014) 28699–28710. DOI:10.1021/jp5096695 |
| [37] | Martín C.F., Stöckel E., Clowes R., et al. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J. Mater. Chem. 21 (2011) 5475–5483. DOI:10.1039/c0jm03534c |
| [38] | Koyuncu S., Gultekin B., Zafer C., et al. Electrochemical and optical properties of biphenyl bridged-dicarbazole oligomer films:electropolymerization and electrochromism. Electrochim. Acta 54 (2009) 5694–5702. DOI:10.1016/j.electacta.2009.05.014 |
| [39] | Rabbani M.G., El-Kaderi H.M.. Template-free synthesis of a highly porous benzimidazole-linked polymer for CO2 capture and H2 storage. Chem. Mater. 23 (2011) 1650–1653. DOI:10.1021/cm200411p |
 2016, Vol. 27
2016, Vol. 27