b Department of Chemistry, College of Sciences, Shanghai University, Shanghai 200444, China
In recent decades, peroxide explosives (PEs) are becoming more and more noticeable as one class of the most elusive explosives to individual health [1, 2], social stability [3] and terrorism activities [4]. From hydrogen peroxide (H2O2) to organic peroxides (Table 1), such as triacetone triperoxide (TATP), diacetone diperoxide (DADP) and hexamethylene triperoxide diamine (HMTD), which can be easily made from commercially available precursors (H2O2, an acid, and acetone). TATP is one of the most sensitive explosives known, which has 88% explosive equivalence of TNT [5]. Well- known terrorist attacks using TATP as bombs including Richard Reid’s shoe bombing on December 22, 2001 [6], the London bombings on July 7, 2005 [7], and the suicide bombers on the evening of 13th November 2015 in Paris and the explosions at the airport and the metro station in Brussels on Tuesday 2th March 2016 [8]. H2O2 is considered as an intrinsic impurity, which can be generated via UV decomposition or simply leaking of the PEs [9]. It’s worth noting that the mixtures of H2O2 and alcohols or acetone can be used as powerful explosives as well [10]. Thus, trace the detection of H2O2 vapor is crucial for security scenarios [11].
|
|
Table 1 Compounds of interest for explosive detection [33]. |
The first method for quantitative trace analysis of peroxide- based explosives is described by Karst et al. [12] using reversed- phase high-performance liquid chromatography method with postcolumn UV irradiation and fluorescence detection for the analysis. After that, there are various methods and successful technologies used to report about detection the PEs, such as the electrochemical [13-16], voltammetric [17, 18], electrogenerated chemiluminescence (ECL) [19, 20], spectrophotometry [21, 22], thermodynamic [23], differential scanning calorimetry (DSC) and thermogravimetric analyzer (TG) [24], high-performance liquid chromatography [25, 26], mass spectrometry (MS) and ion mobility spectrometry (IMS) [27-29] and so on. In spite of several presented methods of sensing PEs, they generally needed large instrument, a complex pretreated process, long operation time, and low sensitivity [30]. As a contrast, fluorescent technique is a current efficient method with simplicity, high sensitivity, selectivity and fast response to explosive detection [31, 32].
Compared to nitroaromaticexplosives, e.g. 2, 4, 6-trinitrotoluene (TNT), the PEs are extremely difficult to detect by conventional techniques owing to their lack ofUV-vis absorbance, fluorescence, a nitro group, or facile ionization that can benefit intermolecular π-π stacking interactions with electron-rich chromophores. Moreover, sensitivity to mechanical stress, easily decomposition and so on also makes the sensing of the PEs more challenging. In this short review, we will discuss the sensing mechanisms of fluorescence based the PEs detection, summarize recent progress on the fluorescence probes in the nearly 5 years, and list the design strategies of the material structures to enhance the sensitivity or selectivity.
2. Signal mechanismsAs mentioned above, fluorescence-based explosives detection is an indirect method which utilizes the interaction of fluorescent sensory materials and explosive to trigger a fluorescence signal change, and hence detects the presence of the explosive.
There are several fluorescent phenomena generated by the concentration and exposure time of explosives in the sensing process, such as intensity (quenching or enhancement), wavelength, or lifetime. In this review, the following parts summarized three kinds of sensing mechanisms commonly used for PEs probe design.
2.1. Boronate oxidation reactionAmong all the reported fluorescent PEs probes, the probe based on boronate oxidation reaction dominates. Chemical reaction between the boronate group and H2O2 results in obvious fluorescence changes due to the transformation of the molecule structure. The high selectivity of the boronate oxidation reaction has been recognized by previous studies [34].
H2O2 possesses ambiphilic reactivity. On the one hand, its labile O-O bond allows it to react as a two-electron electrophilic oxidant. On the other hand, because of the a-effect of adjacent nonbonding orbitals on its oxygen atoms makes H2O2 be a good nucleophile [35, 36].
Upon reaction with H2O2, aryl boronates act as an electrophile in a reversible manner with nucleophiles to form a negatively charged tetrahedral boronate complex. After that, the C-B bond becomes reactive as a nucleophile (Fig. 1). The reaction of masked boronates with H2O2 is released to form aromatic phenols as functional groups. To improve the efficiency of these characteristic molecular features, we could utilize this dual-mode complementary ambiphilic reactivity of H2O2 with boronates to achieve sensitivity and selectivity, so that this single reaction could endow a plenty variety of fluorescent molecules to realize the detection of H2O2.

|
Download:
|
| Figure 1. The sensing mechanism of boronates as fluorescent probe to detect H2O2. | |
2.2. Specific aldehyde oxidation reaction
Recently, our group [37] designed a highly efficient multiformyl phenol/amine system for fluorescence detection of H2O2 vapor (Fig. 2.). The hydroxyl group together with three formyl units was used to construct a donor-π-acceptor fluorescent molecular probe. Then, the hydroxyl group of the probe reacted with diethylamine to form the ionized molecule. Experimentally, the aldehyde groups of the ionized molecule were either partially or completely oxidized to obtain the carboxylic phenol upon reaction with PEs vapor. Furthermore, the oxidized carboxylic products could form H2O2-related multiple hydrogen bonds, the intermolecular H-bonding interactions is beneficial for a more efficient adsorption capacity H2O2 vapor, which boost response speed, and enhance sensitivity.
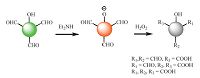
|
Download:
|
| Figure 2. The sensing process of multi-formyl phenol-amine system as fluorescent probe to detect H2O2. | |
The sensing mechanism is far better than that of boronate oxidation reaction mechanism in PEs sensing whatever in sensitivity or response rate. It also provided a new prospective method to design more efficient PEs vapor sensors via utilizing a reaction product to strengthen the interaction between the object analyte and the sensing material.
2.3. Sulfoxide profluorophoresUnlike previously described mechanisms, a special strategy was proposed by Malashikhin et al. [38] using aromatic sulfoxide reagents for visible fluorescence detection of nmol-quantities of TATP. The aromatic sulfoxide fluorophore emission was modulated by the oxidation of an adjacent heteroatom.
Sulfones 1c-3c was more fluorescent than the corresponding sulfoxides, which provided an opportunity for oxidation-based visual TATP detection (Fig. 3). For example, a 50-fold emission increase of 3c relative to 3b was observed and could be easily discerned by naked eye. A visual response to as little as 100 nmol of TATP could be generated after 90 min. Fluorescent signaling based on sulfoxide profluorophore only required brief photolysis, and it was more sensitive than the visual colorimetric detection method [39]. With regard to broader impact, aryl sulfoxides can have application in the detection of species beyond oxidants of interest.
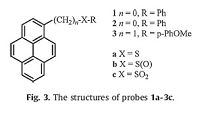
|
Download:
|
| Figure 3. The structures of probes 1a-3c. | |
3. Solution systems probe 3.1. Biological systems
Boronate oxidation reaction could well differentiate H2O2 from other biologically relevant reactive oxygen species (ROS) including superoxide (O2-), hypochlorous acid (HOCl), alkyl peroxides (ROOH), and hydroxyl radical ( OH), therefore it is extensively used as noninvasive detection method in biological systems.
Qian et al. [40] reported the synthesis and photophysical properties of a ratiometric probe 4 (Fig. 4). The boronate-based benzyl cleavable group was attached to the 4-position of 6- dimethylamino-2-methyl quinolinone (DQHP) for detecting H2O2. The probe 4 could be protonated in neutral aqueous solution and formed a charge delocalized state due to the resonance charge transfer from the oxygen atom to quinolinic nitrogen atom. The fluorescence quenching at 542 nm appeared and yielded a new and significant peak maximum at 480 nm once H2O2-triggered boronate cleavage process. Hence, the remarkable changes in the ratio of the emission intensity can be established. Moreover, the good chemoselectivity for H2O2 and pH independence in the biological pH range make the probe 4 a good candidate for detecting H2O2 in aqueous.

|
Download:
|
| Figure 4. The structures of probes 4-9. | |
Xu et al. [41] designed and synthesized a type of BODIPY derivatives including probe 5, 6, 7 (Fig. 4). Among them, the charged structure of probe 7 is beneficial for and intracellular and in vivo sensing due to its easier penetrating into cytomembrane. There is a good linearity between the fluorescence intensity and the concentrations of H2O2 in the range of 0.1-40 μmol/L with a detection limit of 0.03 μmol/L. This work disclosed a new approach toward monitoring and imaging of H2O2 both in vitro and in vivo.
Shen and co-works [42] developed a water-soluble carbazole- derived fluorescent probe 8 (Fig. 4), which showed a fast response, high sensitivity and high selectivity toward H2O2 over other biological reactive oxygen species. Probe 8 exhibited a large (80 nm) red-shifted emission upon the addition of H2O2, which could also be used for detecting H2O2 by a fluorescence quenching method. The probe 8 gave a linear response to H2O2 concentration in the range of 1.0 × 10-8-2.0 × 10-5 mol/L and a detection limit down to 6 nmol/L was obtained.
A charge transfer based fluorescent probe 9 (Fig. 4) was synthesized by Kumar et al. [43], which reported a change in fluorescence emission at two different wavelengths in the presence of H2O2. Once added H2O2 to the solution of receptor 9, the absorbance band (400 nm) decreased accompanying with the appearance of a new absorption band at 522 nm with a solution color change from pale yellow to pink. Furthermore, probe 9 could also be used as a fluorescent probe for intracellular imaging of H2O2 with a change in fluorescence emission from red to blue, which will assist in the exploration of biological processes at a molecular level.
The reported fluorescent probes used for biological detection are generally exhibited a very slow response (sometime one hour or so), which could not match the onsite and quick detection request of the PEs. So, more sensitive and quick fluorescent probes are in great demand.
3.2. Organic solution systemsFluorescent signalling-based methods utilizing fluorescence turn-on in solution after interaction with H2O2 or TATP have been developed to image living cells as well as to detect peroxide recently [44, 45]. A few recent papers reported on fluorescence sensors that can be employed for detection of PEs in aqueous solution.
For example, Germain etal. [46] introduced a fluorescence turnon probe 10 (Fig. 5) targeting PEs (H2O2 and TATP) by using a chelator formed via reaction with H2O2, which was limited to the liquid phase detection of TATP, H2O2, and benzoyl peroxide at a level of 10 nM.

|
Download:
|
| Figure 5. The structures of probes 10 and 11. | |
4, 4-Difuoro-4-bora-3a, 4a-diaza-s-indacene (BODIPY) derivatives with excellent optical properties have been considered to modify for serving as reaction-based chemosensors [47]. In 2014, Matsumoto and co-workers [48] designed a new BODIPY probe 11 (Fig. 5). The probe 11 showed a maximal absorption peak at 621 nm (ε = 8.74 × 104 L mol-1 cm-1) with blue color in THF solution and a red fluorescence emission bands (λmax = 643 nm). The composition of 11 and tetrabutylammonium hydroxide (TBAH) was spin-coated on the thin layer chromatography (TLC) plate to examine the detection of H2O2 vapor via the red emission (ΔR) change along with the vapor concentration of H2O2. Experimental results showed the detection limit of H2O2 vapor could be estimated 8.43 ppb lower than the value of the permissible exposure limit (OSHA).
4. Thin film fluorescent probeSolution probes for PEs often need longer operation times, and may suffer from reagent contamination. Thin film fluorescent probe is highly favored considering that it could detect the PEs vapor directly by without a contact with the PEs solid or solution and hence no reagent contamination problem. Because the vapor pressures of the PEs are low, and much stronger intermolecular interaction is required to capture the PEs molecules in vapor phase and to guarantee an adequate fluorescence change, thus a direct detection of its vapor is challenging. In addition, the detection should show a quick response, high sensitivity and facile operation, etc. Due to the difficulty, the pioneering fluorescence sensors that can be employed for vapor detection of H2O2, both suffer from long response time and complicated instrument alignment [49]. It is an urgent need to develop highly efficient and reliable fluorescence PEs sensory materials and detector systems.
4.1. Inorganic small moleculeAlthough vapor detection of H2O2 still remains challenging, Zhang et al. [50] reported a gas sensor based on In2O3 nanoparticles, which is capable of detecting TATP in the range of 0.5-500 mg powder, corresponding to 2.9 ppb-2.8 ppm.
Recently, Xu et al. [51] developed an efficient colorimetric sensing probe 12 coated on the cellulose microfibril network of paper towels, which provided a tunable interface for modification with Ti(IV) oxo complexes for binding and reacting with H2O2 (Fig. 6). Experimentally, the Ti(IV)-peroxide bond formed that turned the complex from colorless to bright yellow with an absorption maximum around 400 nm. The colorimetric sensor system could detect H2O2 vapor down to ppb level. Such a cheap and simple approach provided plenty of opportunities to enlarge the surface area (by shrinking the fiber size), enhancing the surface interaction with gas phase.
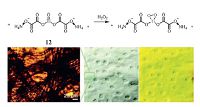
|
Download:
|
| Figure 6. (Top) Colorimetric sensing based on peroxide complexation with Ti(IV) oxo moiety (>Ti O). (Bottom) Yellow color formation as photographed over a piece of paper towel (2 × 2 cm2, loaded with 0.1 mmol titanyl oxalate) upon exposure to the vapor of 35 wt% H2O2 solution. Also shown is an optical microscope photograph of the paper towel, revealing the cellulose fibril network. Reprinted with permission from Xu et al. [51], Copyright 2011, American Chemical Society. | |
4.2. Organic small molecule fluorophores
With the early development of fluorescence-based explosive sensors, small molecular fluorophores have become a big part of the sensors. Considering their simple synthesis and varied pathways of fluorescence change, various fluorescent probes can be designed to detect a wide range of object explosives.
In 2013, Xu et al. [52] reported a fluorescence turn-on probe 13 (Fig. 7) for the detection of H2O2 vapor, in which electron deficient group boronate was attached with naphthalimide backbone to get command of the "push-pull" electronic structure of the probe. To provide the appropriate basic reaction conditions, a mixture of 13 and TBAH was casted onto the TLC plate for the H2O2 vapor detection.
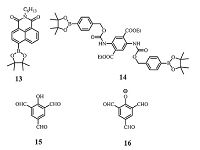
|
Download:
|
| Figure 7. The structures of probes 13-16. | |
Probe 13 could show a maximum absorption peaks at 392 nm with weakly fluorescence. After interaction with H2O2 vapor, the maximum absorption peak of the ultimately oxidation product is red-shifed by 90 nm and turned out to be strongly fluorescent owning to the charge transfer transition. These results illustrated that the intramolecular charge transfer (ICT) transition occurred between the phenol and naphthalimide groups. The detection limit of the sensor material 9 was calculated to be 2.9 ppb and response time was revealed down to 1 s under 1 ppm H2O2.
Xu et al. [53] also developed a ratiometric fluorescence sensor molecule 14 (Fig. 7) for trace vapor detection of H2O2. The probe 14 improved sensitivity and reliability in stoichiometric response. To speed up the sensing reaction, TBAH should also be mixed with 14 to fabricate the sensing film.
The solid film of 14 was fluorescent peaked at 500 nm, while the emission peaks of the phenol product red-shifted to 574 nm. The significant spectral overlap between the absorption band of product (served as acceptor) and the emission band of 14 (served as donor) enabled efficient Förster resonance energy transfer (FRET), which could increase the quenching efficiency by monitoring the fluorescence change at 500 nm. The dual channel (wavelength) monitoring could enhance higher reliability and effectiveness of detection H2O2 vapor than conventional single wavelength monitoring (fluorescence turn-on or off). The detection limit of sensor material 14 for H2O2 could be projected to be as low as 7.7 ppb and fast sensor response revealed down to 0.5 s under 1 ppm of H2O2.
Very recently, our group [37] reported a highly efficient multiformyl phenol-amine system for fluorescence detection of the PEs vapor. The probe is very simple in structure, and it could be easily synthesized and scaled up. It is composed of a hydroxyl group and three aldehyde groups at the 2, 4, 6 position of phenyl unit (probe 15) . It is interesting that 15 (Fig. 7) showed no response to PEs vapor, but upon reaction with diethylamine to afford TFP-I 16 (Fig. 7), the probe demonstrated excellent photo-stability, boosted response speed and enhanced sensitivity to PEs vapor. The most exquisite point of the probe is that oxidized product of the sensing process could form multiple hydrogen bonding with PEs vapor, which could strengthen the capture of the PEs vapor and enhance the sensing performance.
As comparison, the quenching rate of 15 film is only 18% within 300 s to contact the H2O2 vapor, while the 16 film performed a much better response rate, 85% quenching during the first 50 s. Time-related fluorescence quenching curves of 16 in the PEs vapor presented a 42% quenching in DADP and 29.3% in TATP within 300 s. The detection limit could be as low as 0.1 ppt for H2O2 vapor and 0.2 ppb for TATP (Fig. 8), which is the most sensitive fluorescent probe of PEs at present. Compared with the boranate reaction, the synthesis of this probe did not use expensive organic lithium reagent and low temperature reaction or expensive organo-metalic catalyst, and for the thin film fabrication, no strong organic base such as TBAH is added, which could avoid the phase separation, complicated compositions optimization and influence of oxygen in the air.

|
Download:
|
| Figure 8. Linear fit of (a) H2O2 and (b) TATP concentration-quenching rate plot. The data for H2O2 and TATP quenching are collected at 300 s and 100 s, respectively. Reprinted with permission from Xu et al. [37], Copyright 2015, Royal Society of Chemistry. | |
4.3. Conjugated fluorescent polymers
Fluorescent conjugated polymers (CPs) have recently been used successfully in explosive detection [54], which have an extended exciton migration pathway and efficient electronic communication between detection objects along the backbone in compared with small molecule fluorophores.
Sanchez et al. [55] proposed a linear non-conjugated polymer probe 17 (Fig. 9) based on a turn-on fluorescence for H2O2 vapor detection. The synthesis of poly-3', 6'-bis(1, 3, 2-dioxaborinane)-fluoran 17 was by double transesterification polymerization of 3', 6'-bis(pinacolatoboron)fluoran and pentaerythritol. The six- membered boronic ester rings was applied as a surface detection method for H2O2 vapor by a boronate oxidation reaction. Detection limits as low as 3 ppb over an 8 h period can be achieved with a porous probe film. Experimentally, a spot test of 30 ppm H2O2 in suspicious liquids showed a visual detection within 30 s and the detection limit is 7.7 ppb after 5 min.
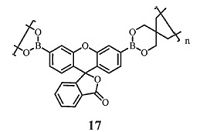
|
Download:
|
| Figure 9. The structures of probes 17. | |
Our group [56] proposed a hyperbranched conjugated polymer probe 18 (Fig. 10). The borate ester units were attached as the periphery units. The merits are that the hyperbranched polymer with a higher generation will show a spherical conformation, thus the borate ester units will be located on the surface of the sphere, which will increase the contact area with the PEs vapor and enhance the sensing performance. For comparison, two linear polymers with a borate ester on the fluorenyl 19 (Fig. 10) or pyrenyl units 20 (Fig. 10) were also synthesized.

|
Download:
|
| Figure 10. The structures of probes 18-20. | |
By spin-coated toluene solution (10-4 mol/L) onto ZnO nanorod array substrate (Fig. 11), the sensing films were fabricated. Experimental results showed that the polymer 18 has larger steric hindrance, higher HOMO level, number of internal cavities and external boron ester groups of the hyperbranched structure, which make it higher sensitivity to H2O2 and TATP vapor than polymer 19 and 20. The fluorescence of 18 was quenched by 60% and 30% of H2O2 and TATP within 300 s upon exposure to vapor. The detection limit of H2O2 could be estimated to be as low as 1.6 ppb.

|
Download:
|
| Figure 11. SEM images of 18-20 films prepared with toluene solvent (all concentrations, 10-3 g/mL) and ZnO nanorod array as film substrate (a: 18; b: 19; c: 20; d: ZnO nanorod array). Reprinted with permission from Chen et al. [56], Copyright 2015, Royal Society of Chemistry. | |
4.4. Colorimetric sensor array
PEs have neither nitro groups or aromatic units, so that PEs are difficult to interact with electron-rich chromophores by intermolecular π-π stacking interactions. Even redox sensitive dyes are not very responsive to TATP vapor. By using a solid acid catalyst to pretreat a gas stream, a colorimetric sensor array was proposed to detect very low levels of decomposition products (e.g. H2O2) of TATP [57].
Herein, Lin et al. [58] reported a colorimetric sensor array using chemical reaction between an array of redox sensitive dyes and acid decomposition products (H2O2) of TATP (Fig. 12a). Using an ordinary flatbed scanner, the red, green, and blue values of each spot in the array were measured before and after exposure to TATP vapor at various concentrations (Fig. 12b). The simple colorimetric sensor could detect TATP vapor semi-quantitatively from 50 ppb to 10 ppm with a detection limit below 2 ppb. The array is highly selective for TATP, and could differentiate TATP from other chemical oxidants (e.g. H2O2, bleach, tert-butylhydroperoxide, peracetic acid).

|
Download:
|
| Figure 12. (a) Acid catalyzed decomposition of TATP. (b) Color difference maps of TATP vapor at concentrations specified after 5 min (top row) and 10 min (bottom row) of exposure at 50% relative humidity and 298 K. For display purposes, the color range of these difference maps is expanded from four to eight bits per color (RGB range of 4-19 expanded to 0-255) . Reprinted with permission from Lin & Suslick [58], Copyright 2010, American Chemical Society. | |
5. Typical strategies to enhance sensitivity
The discussion above is focused on the molecular structure design of the probe, but usually the sensitivity of the probe still could not match the request of the onsite, quick and sensitive request. A further modification of the molecular structure of the probe will be sure to increase the sensitivity, but it will add more unexpected synthetic difficulty. A combination of the probe with a delicate device structure will be more convenient to enhance the sensitivity by increasing the contact area with the analytes, modulating the light emitting direction, and magnifying the light signal. In this part, we will focus on the typical strategies in the sensing device structure to improve the sensitivity for PEs detection.
5.1. Ordered assembly arrays of ZnO nanordsNanomaterials and -structures have become a research focus because of their excellent chemical and thermal stability to improve the sensitivity of film probe.
A highly efficient fluorescence turn-off method of detecting PEs vapor was found by our group [59]. Probe 21 (Fig. 13) showed excellent response to H2O2 vapor, but the photo stability is too poor, so that it decomposed even in air and will not be suitable for PEs detection. To improve the photostability, probe 22 (Fig. 13) is designed, but the sensing performance is decreased with it. To solve the problem, probe 22 was spin-coated onto an ordered ZnO nanorod array. The ordered ZnO nanorod substrate was used both as photo-oxidation catalysts and light modulation. The reaction rate of the 22 film on a ZnO nanorod array was 42-fold faster than that on the quartz plate or in solution. Herein, the fluorescence of the 22 film was 60% quenched within 10 s and the detection limit of TATP vapor was estimated to be 0.5 ppm. These features made it an ideal candidate for chemical detection and analysis in public safety and environmental monitoring.

|
Download:
|
| Figure 13. The structures of probes 21-23. | |
5.2. Fluorophores functionalized on nanoparticles and nanofibers
A carbon dot-based fluorescence turn-on sensor for H2O2 with a photo-induced electron transfer mechanism was developed by Lan etal. [60], which exhibited rapid response to H2O2 with a detection limit of 84 nmol/L. Alternatively, Can et al. [61] presented a novel colorimetric method using Fe3O4 magnetic nanoparticles and converted into a sensor on a Nafion membrane for TATP. Hence, the combination of nanoparticle and fluorophores could be considered as an efficient method to increase sensitivity.
Fluorophores functionalized on nanofibers were also investigated. For example, an array of hybrid organic-semiconductor sensors was developed by Capua et al. [62] aimed at selectively detecting TATP, which could detect it in a concentration of less than 100 ppb. Our group [63] reported the first use of organic semiconductors (OSCs)-coated polyacrylonitrile (PAN) electrospun nanofibrous mats for highly sensitive chemosensors via evanescent-wave guiding effect. Probe 21 and 23 (Fig. 13) were coated onto the nanofibers fabricated by electrospinning. In consideration of the diameters of the fibers were small enough, which leaded to high area-to-volume ratio, excellent gas permeability and the evanescent-wave effect to highly responsive fluorescence quenching-based chemosensors for H2O2 vapor. Experimentally, 9-fold fluorescence intensity, and even 14-fold of sensitivity enhancement were achieved in the sensing of H2O2 vapor by introducing the nanofibers structure. In addition, the fluorescence intensity and sensitivity of the resulting materials were inversely proportional to the diameter of nanofibers. The nanofibers-enhanced detection strategies coated by other functional materials could be extended to more general research fields including toxicity chemical and environmental detection.
6. ConclusionIn recent decades, the accurate and sensitive sensing of the PEs has become more and more noticeable in terms of anti-terrorism, individual health and environmental protection and so on. In this short review, we have summarized some fluorescent probes for the PEs vapors in recent years (Table 2). It could be seen that the probes for PE detection has witnessed a great progress.
|
|
Table 2 Recent fluorescence sensors for the detection of peroxide explosives. |
However, fluorescence-based the PEs sensors face many challenges, such as sensitivity, selectivity and photostability. Therefore, it is desirable to use smart strategy to improve the sensing performance of the PEs probes. Foremost, in order to rationally design the sensing materials, simulation and theoretical studies are necessary to be coupled with material synthesis. Secondly, for long time use detector, photostability is the more important issue for the probe, so it should consider together with the rational molecular design. Thirdly, the surface morphology of the sensing film can affect the sensitivity ofPEs probes. Hence, on the one hand, the morphology of the probe film optimization is very important. On the other hand, the introduction of nano substrate is also a good choice to increase the surface area-to- volume ratio and hence to increase the sensitivity. And for the later issue, the sensing signal modulation with fine structure or smart strategies should also be given a special attention to increase the signal-to-noise ratio and magnify the sensing performance.
The research in this field still needs a much-needed boost to make it use in the onsite explosive detection. For the application, the PEs probes should be low-cost, highly stable, environmental-friendly, and easily be integrated to make a portable and highly efficient PE detector. To develop such detectors, it need the cooperative work of chemists, physists and engineers et al. to integrate vapor collectors, optical path, electric circuit and control units into one portable, efficient and reliable detector. In spite of fluorescence-based, the PEs detection encounters many challenges (sensitivity, selectivity, stability and application), we firmly believe, with current advances in rational design of function materials and progress in mechanism research and cooperation, that fluorescence-based, the PEs probes will have a promising and bright future. Such detectors will soon come to the market and play their critical roles in explosive detection.
Acknowledgment This work was supported by NSFC (Nos. 61325001, 21273267, 61321492, and 51473182).| [1] | G. McDonnell, A.D. Russell. Antiseptics and disinfectants: activity, action, and resistance, Clin. Microbiol. Rev. 12 (1999) 147–179. |
| [2] | S. Tan, Y. Sagara, Y.B. Liu, P. Maher, D. Schubert. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 141 (1998) 1423–1432. DOI:10.1083/jcb.141.6.1423 |
| [3] | J. Roach, P. Ekblom, R. Flynn. The conjunction of terrorist opportunity: a framework for diagnosing and preventing acts of terrorism. Sec. J. 18 (2005) 7–25. |
| [4] | R. Schulte-Ladbeck, M. Vogel, U. Karst. Recent methods for the determination of peroxide-based explosives. Anal. Bioanal. Chem. 386 (2006) 559–565. DOI:10.1007/s00216-006-0579-y |
| [5] | K.F. Ferris, R.J. Bartlett. Hydrogen pentazole: does it exist?. J. Am. Chem. Soc. 114 (1992) 8302–8303. DOI:10.1021/ja00047a058 |
| [6] | Richard Reid's shoe bombing on American Airlines Flight 63 on December 22, 2001. http://en.wikipedia.org/wiki/Richard_Reid. |
| [7] | The London bombings on July 7, 2005. http://en.wikipedia.org/wiki/7_July_2005_London_bombings. |
| [8] | The explosions at the airport and the metro station in Brussels on Tuesday 2th March 2016. http://www.baidu.com/s?ie=utf8&oe=utf8&wd=theexplosionsattheairportandthemetrostationinBrusselsonTuesday2thMarch2016&tn=98010089_dg&ch=3. |
| [9] | J. Wang. Electrochemical sensing of explosives. Electroanalysis 19 (2007) 415–423. DOI:10.1002/(ISSN)1521-4109 |
| [10] | F. Dubnikova, R. Kosloff, J. Almog, et al. Decomposition of triacetone triperoxide is an entropic explosion. J. Am. Chem. Soc. 127 (2005) 1146–1159. DOI:10.1021/ja0464903 |
| [11] | J.C. Sanchez, W.C. Trogler. Efficient blue-emitting silafluorene-fluorene-conjugated copolymers: selective turn-off/turn-on detection of explosives. J. Mater. Chem. 18 (2008) 3143–3156. DOI:10.1039/b802623h |
| [12] | R. Schulte-Ladbeck, P. Kolla, U. Karst. Trace analysis of peroxide-based explosives. Anal. Chem. 75 (2003) 731–735. DOI:10.1021/ac020392n |
| [13] | F.I. Bohrer, C.N. Colesniuc, J. Park, et al. Selective detection of vapor phase hydrogen peroxide with phthalocyanine chemiresistors. J. Am. Chem. Soc. 130 (2008) 3712–3713. DOI:10.1021/ja710324f |
| [14] | W.Z. Jia, M. Guo, Z. Zheng, et al. Vertically aligned CuO nanowires based electrode for amperometric detection ofhydrogen peroxide. Electroanalysis 20 (2008) 2153–2157. DOI:10.1002/elan.v20:19 |
| [15] | S.H. Chen, R. Yuan, Y.Q. Chai, F.X. Hu. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: a review. Microchim. Acta 180 (2012) 15–32. |
| [16] | S.K. Mamo, J. Gonzalez-Rodriguez. Development of a molecularly imprinted polymer-based sensor for the electrochemical determination of triacetone triperoxide (TATP). Sensors 14 (2014) 23269–23282. DOI:10.3390/s141223269 |
| [17] | A.M. Smolin, N.P. Novoselov, T.A. Babkova, S.N. Eliseeva, V.V. Kondrat'ev. Use of composite films based on poly(3, 4-ethylenedioxythiophene) with inclusions of palladium nanoparticles in voltammetric sensors for hydrogen peroxide. J. Anal. Chem. 70 (2015) 967–973. DOI:10.1134/S1061934815080171 |
| [18] | Y.Q. Xie, I.F. Cheng. Selective and rapid detection of triacetone triperoxide by double-step chronoamperometry. Microchem. J. 94 (2010) 166–170. DOI:10.1016/j.microc.2009.10.016 |
| [19] | A. Shaw, P. Lindhome, R.L. Calhoun. Electrogenerated chemiluminescence (ECL) quenching of Ru(bpy)32+ by the explosives TATP and Tetryl. J. Electrochem. Soc. 160 (2013) H782–H786. DOI:10.1149/2.005311jes |
| [20] | S. Parajuli, W.J. Miao. Sensitive determination of triacetone triperoxide explosives using electrogenerated chemiluminescence. Anal. Chem. 85 (2013) 8008–8015. DOI:10.1021/ac401962b |
| [21] | Y. Sang, L. Zhang, Y.F. Li, et al. A visual detection of hydrogen peroxide on the basis of Fenton reaction with gold nanoparticles. Anal. Chim. Acta 659 (2010) 224–228. DOI:10.1016/j.aca.2009.11.031 |
| [22] | Ş. Eren, A. Üzer, Z.Y. Can, et al. Determination of peroxide-based explosives with copper(II)-neocuproine assay combined with a molecular spectroscopic sensor. Analyst 135 (2010) 2085–2091. DOI:10.1039/b925653a |
| [23] | M. Amani, Y. Chu, K.L. Waterman, et al. Detection of triacetone triperoxide (TATP) using a thermodynamic based gas sensor. Sensor. Actuat. B Chem. 162 (2012) 7–13. DOI:10.1016/j.snb.2011.11.019 |
| [24] | S.H. Wu, I.J. Wen, C.C. Chiang, et al. Effects of various fire-extinguishing reagents for thermal hazard of triacetone triperoxide (TATP) by DSC/TG. J. Therm. Anal. Calorim. 113 (2013) 991–995. DOI:10.1007/s10973-012-2788-2 |
| [25] | S.M. Steinberg. High-performance liquid chromatography method for determination of hydrogen peroxide in aqueous solution and application to simulated Martian soil and related materials. Environ. Monit. Assess 185 (2013) 3749–3757. DOI:10.1007/s10661-012-2825-4 |
| [26] | M. Tarvin, B. McCord, K. Mount, K. Sherlach, M.L. Miller. Optimization of two methods for the analysis of hydrogen peroxide: high performance liquid chromatography with fluorescence detection and high performance liquid chromatography with electrochemical detection in direct current mode. J. Chromatogr. A 1217 (2010) 7564–7572. DOI:10.1016/j.chroma.2010.10.022 |
| [27] | M.E. Sigman, C.D. Clark, R. Fidler, C.L. Geiger, C.A. Clausen. Analysis of triacetone triperoxide by gas chromatography/mass spectrometry and gas chromatography/tandem mass spectrometry by electron and chemical ionization. Rapid Commun. Mass Spectrom. 20 (2006) 2851–2857. DOI:10.1002/(ISSN)1097-0231 |
| [28] | R.M. Räsänen, M. Nousiainen, K. Peräkorpi, et al. Determination of gas phase triacetone triperoxide with aspiration ion mobility spectrometry and gas chromatography-mass spectrometry. Anal. Chim. Acta 623 (2008) 59–65. DOI:10.1016/j.aca.2008.05.076 |
| [29] | A. Kende, F. Lebics, Z. Eke, K. Torkos. Trace level triacetone-triperoxide identification with SPME-GC-MS in model systems. Microchim. Acta 163 (2008) 335–338. DOI:10.1007/s00604-008-0001-x |
| [30] | R.M. Burks, D.S. Hage. Current trends in the detection of peroxide-based explosives. Anal. Bioanal. Chem. 395 (2009) 301–313. DOI:10.1007/s00216-009-2968-5 |
| [31] | X.C. Sun, Y. Wang, Y. Lei. Fluorescence based explosive detection: from mechanisms to sensory materials. Chem. Soc. Rev. 44 (2015) 8019–8061. DOI:10.1039/C5CS00496A |
| [32] | M.E. Germain, M.J. Knapp. Optical explosives detection: from color changes to fluorescence turn-on. Chem. Soc. Rev. 38 (2009) 2543–2555. DOI:10.1039/b809631g |
| [33] | H. Östmark, S. Wallin, H.G. Ang. Vapor pressure of explosives: a critical review. Propell. Explos. Pyrotech. 37 (2012) 12–23. DOI:10.1002/prep.v37.1 |
| [34] | A.R. Lippert, G.C. van de Bittner, C.J. Chang. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 44 (2011) 793–804. DOI:10.1021/ar200126t |
| [35] | W.P. Jencks, J. Carriuolo. Reactivity of nucleophilic reagents toward esters. J. Am. Chem. Soc. 82 (1960) 1778–1786. DOI:10.1021/ja01492a058 |
| [36] | Y. Ren, H. Yamataka. The a-effect in gas-phase SN2 reactions: existence and the origin of the effect. J. Org. Chem. 72 (2007) 5660–5667. DOI:10.1021/jo070650m |
| [37] | W. Xu, Y.Y. Fu, Y.X. Gao, et al. A simple but highly efficient multi-formyl phenolamine system for fluorescence detection of peroxide explosive vapour. Chem. Commun. 51 (2015) 10868–10870. DOI:10.1039/C5CC03406J |
| [38] | S. Malashikhin, N.S. Finney. Fluorescent signaling based on sulfoxide profluorophores: application to the visual detection of the explosive TATP. J. Am. Chem. Soc. 130 (2008) 12846–12847. DOI:10.1021/ja802989v |
| [39] | R. Schulte-Ladbeck, P. Kolla, U. Karst. A field test for the detection of peroxidebased explosives. Analyst 127 (2002) 1152–1154. DOI:10.1039/b206673b |
| [40] | Y.Y. Qian, L. Xue, D.X. Hu, G.P. Li, H. Jiang. Quinoline-based fluorescent probe for ratiometric detection of hydrogen peroxide in aqueous solution. Dyes Pigments 95 (2012) 373–376. DOI:10.1016/j.dyepig.2012.05.013 |
| [41] | J. Xu, Q. Li, Y. Yue, Y. Guo, S.J. Shao. A water-soluble BODIPY derivative as a highly selective "Turn-On" fluorescent sensor for H2O2 sensing in vivo. Biosens. Bioelectron. 56 (2014) 58–63. DOI:10.1016/j.bios.2013.12.065 |
| [42] | Y.M. Shen, B. Kong, X.F. Peng, et al. A new turn-off fluorescence chemosensor for hydrogen peroxide based on carbazole derivative in aqueous solution. Adv. Mater. Res. 1006-1007 (2014) 821–825. DOI:10.4028/www.scientific.net/AMR.1006-1007 |
| [43] | M. Kumar, N. Kumar, V. Bhalla, P.R. Sharma, Y. Qurishi. A charge transfer assisted fluorescent probe for selective detection of hydrogen peroxide among different reactive oxygen species. Chem. Commun. 48 (2012) 4719–4721. DOI:10.1039/c2cc30932g |
| [44] | B.C. Dickinson, C. Huynh, C.J. Chang. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 132 (2010) 5906–5915. DOI:10.1021/ja1014103 |
| [45] | D. Srikun, A.E. Albers, C.I. Nam, A.T. Iavarone, C.J. Chang. Organelle-targetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-tag protein labeling. J. Am. Chem. Soc. 132 (2010) 4455–4465. DOI:10.1021/ja100117u |
| [46] | M.E. Germain, M.J. Knapp. Turn-on fluorescence detection of H2O2 and TATP. Inorg. Chem. 47 (2008) 9748–9750. DOI:10.1021/ic801317x |
| [47] | Y.Y. Fu, Q.G. He, D.F. Zhu, et al. A BODIPY dye as a reactive chromophoric/ fluorogenic probe for selective and quick detection of vapors of secondary amines. Chem. Commun. 49 (2013) 11266–11268. DOI:10.1039/c3cc46571c |
| [48] | A. Matsumoto, R. Nishiyabu, Y. Kubo. Synthesis of a borylated boron-dibenzopyrromethene dye enabling the visual detection of H2O2 vapor. RSC Adv. 4 (2014) 37973–37978. DOI:10.1039/C4RA06061J |
| [49] | J.Y. Zheng, Y.L. Yan, X.P. Wang, et al. Hydrogen peroxide vapor sensing with organic core/sheath nanowire optical waveguides. Adv. Mater. 24 (2012) OP194–OP199. |
| [50] | W.H. Zhang, W.D. Zhang, L.Y. Chen. Highly sensitive detection of explosive triacetone triperoxide by an In2O3 sensor. Nanotechnology 21 (2010) 315502. DOI:10.1088/0957-4484/21/31/315502 |
| [51] | M. Xu, B.R. Bunes, L. Zang. Paper-based vapor detection of hydrogen peroxide: colorimetric sensing with tunable interface. ACS Appl. Mater. Interfaces 3 (2011) 642–647. DOI:10.1021/am1012535 |
| [52] | M. Xu, J.M. Han, Y.Q. Zhang, X.M. Yang, L. Zang. A selective fluorescence turn-on sensor for trace vapor detection of hydrogen peroxide. Chem. Commun. 49 (2013) 11779–11781. DOI:10.1039/c3cc47631f |
| [53] | M. Xu, J.M. Han, C. Wang, et al. Fluorescence ratiometric sensor for trace vapor detection of hydrogen peroxide. ACS Appl. Mater. Interfaces 6 (2014) 8708–8714. DOI:10.1021/am501502v |
| [54] | S. Rochat, T.M. Swager. Conjugated amplifying polymers for optical sensing applications. ACS Appl. Mater. Interfaces 5 (2013) 4488–4502. DOI:10.1021/am400939w |
| [55] | J.C. Sanchez, W.C. Trogler. Polymerization of a boronate-functionalized fluorophore by double transesterification: applications to fluorescence detection of hydrogen peroxide vapor. J. Mater. Chem. 18 (2008) 5134–5141. DOI:10.1039/b809674k |
| [56] | L. Chen, Y.X. Gao, Y.Y. Fu, etal.. Borate ester endcappedfluorescent hyperbranched conjugated polymer for trace peroxide explosive vapor detection. RSC Adv. 5 (2015) 29624–29630. DOI:10.1039/C5RA02472B |
| [57] | D. Armitt, P. Zimmermann, S. Ellis-Steinborner. Gas chromatography/mass spectrometry analysis of triacetone triperoxide (TATP) degradation products. Rapid Commun. Mass Spectrom. 22 (2008) 950–958. DOI:10.1002/(ISSN)1097-0231 |
| [58] | H.W. Lin, K.S. Suslick. A colorimetric sensor array for detection of triacetone triperoxide vapor. J. Am. Chem. Soc. 132 (2010) 15519–15521. DOI:10.1021/ja107419t |
| [59] | C. He, D.F. Zhu, Q.G. He, et al. A highly efficient fluorescent sensor of explosive peroxide vapor via ZnO nanorod array catalyzed deboronation of pyrenyl borate. Chem. Commun. 48 (2012) 5739–5741. DOI:10.1039/c2cc31386c |
| [60] | M.H. Lan, Y.F. Di, X.Y. Zhu, et al. A carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism. Chem. Commun. 51 (2015) 15574–15577. DOI:10.1039/C5CC05835J |
| [61] | Z.Y. Can, A. Üzer, K. Türkekul, E. Erçağ, R. Apak. Determination of triacetone triperoxide with a N,N-dimethyl-p-phenylenediamine sensor on nafion using Fe3O4 magnetic nanoparticles. Anal. Chem. 87 (2015) 9589–9594. DOI:10.1021/acs.analchem.5b01775 |
| [62] | E. Capua, R. Cao, C.N. Sukenik, R. Naaman. Detection of triacetone triperoxide (TATP) with an array of sensors based on non-specific interactions. Sensor. Actuat. B Chem. 140 (2009) 122–127. DOI:10.1016/j.snb.2009.04.045 |
| [63] | K.Y. Hua, C.M. Deng, C. He, et al. Organic semiconductors-coated polyacrylonitrile (PAN) electrospun nanofibrous mats for highly sensitive chemosensors via evanescent-wave guiding effect. Chin. Chem. Lett. 24 (2013) 643–646. DOI:10.1016/j.cclet.2013.04.033 |
 2016, Vol. 27
2016, Vol. 27 




