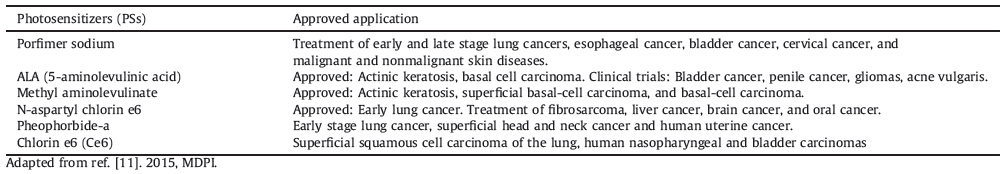
Cancer has been one of the leading causes of death in the world. The conventional cancer treatments mainly include surgery, chemotherapies, radiotherapies, and more recent small molecule-based therapies and immunotherapies [1]. While surgical resection of tumors in many occasions is high recurrence rate, chemotherapy and radiotherapy are associated with systemic side effects to normal tissues and limited by the cumulative radiation dose to cancer cells, respectively. Although it is important to refine the conventional cancer therapy modalities, much effort has also been concentrated on investigating alternative therapy modalities which are safe, effective, affordable, and acceptable to patients. PDT is a noninvasive therapeutic method for cancers, which combines the light of a specific wavelength, a photosensitizer (PS), and oxygen that leads to generation of highly reactive oxygen species (ROS) such as singlet oxygen (1O2) , hydroxyl radical, superoxide anion, and hydrogen peroxide (Fig. 1) [2, 3]. The ROS can cause cellular damage through tumor cell ablation, vascular shutdown, and activation of an immune response against targeted cells that could result in tumor cell death via either necrosis or apoptosis [4-8]. Additionally, since the short diffusion distance and short lifetime of the ROS, the cytotoxicity is limited to the illuminated area containing PSs. Therefore, the selectivity of PS is contributed to PDT with minimal adverse impact on normal tissues, while this is still a great challenge for chemotherapy and radiotherapy [9, 10]. Clearly, compared with traditional cancer therapies, PDT has significant advantages including noninvasive nature, repeatability without cumulative toxicity, the lack of associated side effects, and improved quality of life of the patients. Over the past decades, PDT has been verified to be effective in a varietyofcancers including head and neck [11], lung [12], and skin [13]. On the other hand, PDT has also been applied in the treatment of cardiovascular, dermatological, ophthalmic and infectious diseases [14]. However, the application of PDT in clinic for treatment of cancers or diseases is limited due to lack of tumor selectivity, causing skin photosensitivity, challenges in formulating PS, difficulties in passing through more than few millimeters of tissue, and incapability in treating advanced disseminated diseases [15, 16].
|
Download:
|
| Figure 1. A typical mechanism of PDT cytototoxicity. Adapted from ref. [9]. 2002,Elsevier. | |
Many kinds ofnanoparticles (NPs) have been developed to date, including polymeric NPs, liposomes, inorganic/organic hybrid NPs, which have been widely used to load various diagnostic or therapeutic agents through chemical conjugation or physical entrapment to benefit from the sophisticated nanostructures and large surface area to volume ratios. Consequently, for solving above problems associated with classical PSs, NPs as one of the effective methods have become an advanced technology in the field of PDT that can overcome most of the limitations of classical PSs. NPs-based on PSs decorating specific ligands such as monoclonal antibodies, peptides, or polyethylene glycols could improve the water solubility of PSs, enhance their blood circulation and tumor specific accumulation, and thus improve the therapeutic efficacy and specificity of PDT [17-19]. Therefore, nanotechnology based on the functional NPs could provide a single platform for the integration of multiple functionalities between different components. In particular, this review is focused on organic NPs that have amphiphilic characters enabling selfassembly in aqueous conditions. Various organic nanomaterials such as liposomes, polymeric NPs, natural macromolecule NPs, as well as a number of other functional NPs with interesting chemical and physical properties have been exploited for the delivery of PSs, showing encouraging results in vitro and in vivo [15, 20]. Additionally, organic NPs are often fabricated by noncovalent interactions, which could make them more labile in nature, and provide a route for clearance from the body. Furthermore, the flexibility of organic NPs induces them to change shape or conformation in response to external environmental stimuli, which can be used in detecting of physicochemical changes, molecular binding interactions, and stimuli-driven effects [16]. Thus, nano-formulating PSs incorporated with organic NPs could be a potential strategy to satisfy the requirements of an ideal PDT system. In this review, we summarize several organic PS carriers that have demonstrated for the enhanced efficacy of PDT against cancers.
2. PhotosensitizersPSs are the key factor in PDT. PS can absorb light at a suitable wavelength and cause chemical or physical changes of another chemical entity to generate ROS [8]. An ideal PDT PS is expected to have the ability to preferentially accumulate in tumor tissue and rapidly clear from the normal tissue [21]. It needs to fulfill the following requirements: (1) It should have no dark toxicity, minimal skin photosensitivity and should selectively accumulate in tumor tissue; (2) The PS is stable and soluble in aqueous media; (3) It may be rapidly excreted from the body to reduce systemic toxicity at the end of therapy; (4) It exhibits high quantum yield in cell inactivation. (5) High absorption coefficient in the long wavelength region (700 nm to 800 nm). (6) There should be maximum quantum yield of triplet formation. With so many different requirements, it has a tremendous challenge to find or develop any drug that can confirm to be an ideal PS. However, many PSs that do not satisfy all these requirements have been approved for clinical use while others are under clinical trial, with some others being tested in clinical trial (Table 1), and they largely belong to the first and second generation of PSs [22]. Although the first and second generation PSs have been effective in their clear feasibility and viability of PDT, it has not been optimum with regard to their efficacy. This is because PSs developed for PDT seems to have been driven chemically, rather than clinically. In clinical use, PDT often fails to plan and customize the treatment for the clinicians, partly due to the shortcomings in the properties of PSs, and the incomplete knowledge about the light dose to be delivered for the tumor treatment without affecting the surrounding normal tissue.
|
|
Table 1 List of 1st and 2nd PSs that are approved for clinical use or those in clinical trials. |
Now, some researchers have concentrated on the investigation of the third generation PSs that can be activated with light of a longer wavelength, provoke shorter generalized photosensitivity and have better tumor specificity. This can be acquired through modifying existing PSs with biologic conjugates to realize tumor specific targeting of PSs, or via chemical conjugation/encapsulation of PSs in delivery vehicles or carriers that can improve the efficiency of the drug in blood from the administration site to the target tissue [23]. In brief, the third generation PSs will improve the delivery or targeting abilities of the second generation PSs. In this review, we mainly concentrate on organic NPs constructed by chemical conjugation/encapsulation of PSs.
3. The challenges of PDTThe presence of problem is the incapability of PDT to treat solid, bulky tumors or deep-seated tumors. In addition, the advanced disseminated disease treated by PDT is impossible since whole body illumination is not possible at least with the currently available technologies [24]. Moreover, it is well known that the PDT is an oxygen consuming modality. In other words, the antitumor effects of PDT will be severely affected in the absence of tissue oxygen, since hypoxic tumor cells in solid tumors are PDT resistant [25]. Many factors influence the efficiency of tumors treatment including the chemistry of the PS used, light intensity and wavelength, and oxygen concentration. Therefore, much attention is urgently needed to resolve the problems of the PS or modify the existing PS to overcome the difficulties that might be the potential obstacle to the clinical application of PDT.
Various strategies have been developed to overcome the current deficiencies regarding the issues of the PSs, often by applying a delivery vehicle that enables a stable dispersion of PSs in aqueous solution [15]. Despite enhanced drug loading and increased tumor uptake over free drugs, the traditional delivery systems applying low molecular weight surfactants have been reported to induce severe hypersensitivity reactions in vivo. Therefore, more stable, biocompatible and tumor selective carrier systems are required for efficient delivery of PSs.
4. Organic NPs as delivery vehicles of PSsNPs as an emerging technology have presented the capability to overcome most of the limitations of classical PSs in the field of PDT. NPs, defined as submicroscopic particles between 1 nm and 200 nm in size, have obviously physical, chemical, and structural properties that enable them to widely apply in drug delivery, medical imaging, diagnosis and treatment [26]. The requirements of an ideal PS can be achieved by constructing NPs-based PSs delivery systems. In these systems, PSs are either encapsulated in NPs through covalent/non-covalent interactions, which have at least some of the following advantages [27, 28]:
(1) High surface to volume ratio and the possibility of high drug loading.
(2) The enhanced permeability and retention (EPR) effect.
(3) Targeting potential enhances PS concentration at the desired site and reduces toxic effects toward normal tissues/ cells.
(4) Improving the solubility, biodistribution, pharmacokinetics, cell uptake, and targeting abilities of the PSs.
(5) Maintaining a constant therapeutic dose at the site of action.
(6) Development of multifunctional nanoplatforms in order to carry multiple components, for example, imaging agents, chemotherapeutic drugs, and targeting ligands [29].
These PS carriers can be further classified into organic and inorganic NPs. Here, this paper would comprehensively review the recent advances regarding the development of PDT for cancer treatment using functional organic NPs.
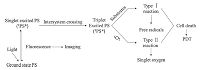
4.1. Liposomal NPsLiposomes are synthetic lipid vesicles made up of one or more concentric phospholipid bilayers composed of natural or synthetic lipids [18]. Liposomes have both a hydrophilic and hydrophobic region, thereby permitting the encapsulation and transport of both hydrophilic PS in their aqueous core or hydrophobic PS within their lamellae [30]. Several studies have shown strong evidence that liposomal formulation of PS is effective in PDT. Zheng group discovered the first all-organic nanoparticle porphysomes, which were bilayer vesicles self-assembled from porphyrin-lipid. The very high porphyrin packing density leaded to structure-dependent "super"-quenching, which hence generated only a small amount of ROS upon excitation. However, the degradation of the porphysome structure resulted in an improvement in PS activity because of photophysical unquenching (Fig. 2A and 2B) [31, 32]. The advantages of PDT could be exhibited for treating tumor when degradation occurred within a cellular compartment of a tumor.
|
Download:
|
| Figure 2. (A) Schematic representation of a pyropheophorbide-lipid porphysome. The phospholipid headgroup (red) and porphyrin (blue) are highlighted in the subunit (left) and assembled nanovesicle (right). (B) Electron micrographs of negatively stained porphysomes. Reproduced with permission from ref. [31]. Copyright 2011 Nature Publishing Group. (C) Formation of PBCs from a PORSIL lipid. Reproduced with permission from ref. [44]. Copyright 2011 Wiley-VCH. | |
Although liposomes have proved to be biocompatible carriers of PS, they did not emerge as an ultimate PS delivery system. The conventional liposomes could be quickly removed from the circulation by cells of the membrane-permeable sequence (MPS) and also prone to disintegration. The resulting short circulation time of unmodified liposomes makes it difficult to achieve elevated tumor-to-normal tissue ratios of PSs [33, 34]. Therefore, liposomes with a specifically modified design, like poly(ethylene glycol) (PEG) grafted long-circulating liposomes [35] or antibody [36], peptide [37], or folic acid (FA) [38] can be designed and prepared for enhanced pharmacokinetic properties as well as improved therapeutics. The most commonly applied ligand is PEG, a hydrophilic polymer that curtails recognition and is uptaked by the reticuloendothelial system (RES) to maintain effective blood concentration. These modified liposomes have exhibited excellent protein resistance, minimum toxicity and non-immunogenicity. Previously, Zheng [39] and Skupin-Mrugalska [40] have done a lot of excellent works in terms of liposomes with a specifically modified design. For example, Jin et al. investigated the use of FA- conjugated porphysomes for targeted PDT which leaded to a significant reduction in cell viability as compared to the untargeted control porphysomes with 671 nm light treatment [38]. Herein, active targeting of liposome was anticipated to increase the drug delivery and overall PDT efficacy by improving tumor selectivity of the PS, but the results indicated the PS concentration in liposomes was generally less than 10%.
According to previous reports, liposomes have also been studied with a triggered release mechanism, so that the release of the PSs could be effectively controlled. Various stimuli, such as heat, light, pH, and enzymes have gained more attention because of their potential in being truly tumor tropic carriers [41, 42]. This mechanism has seemed to enhance cellular internalization of the PS which consequently contributes to increase tumor accumulation and minimizes the nonspecific photosensitivity of normal tissues. Moreover, multifunctional liposomal formulation for theranostic application such as nanocells [43] and the cerasomes [44] has been developed. While, nanocells could be formed from PSs incorporated polymeric NPs encapsulated in a PEGylated liposome, cerasomes have been developed by chemical conjugation of porphyrin to an organoalkoxysilylated lipid. These novel liposomes exhibited the potential in drug delivery capabilities, multimodal imaging, and multimodal therapeutics. For example, Liang et al. reported multifunctional liposomal carrier named cerasome with various advantages including high drug loading efficiency of33.46%, high stability, preventing premature release of PSs during systemic circulation, and exhibiting good fluorescence even at an extremely high number of porphyrins compared with the conventional liposomes (Fig. 2C) [44].
4.2. Polymer-based NPsPolymeric NPs have been widely exploited for their chemical and physical properties and applied in the delivery of PSs in PDT [45, 46]. These polymeric NPs have offered several key advantages over molecular PDT drugs and could be formulated to transport PSs in a controlled and targeted fashion through further surface modification with specific ligands [47]. Moreover, the size of polymeric NPs could enable them as an appropriate tool for drug delivery via EPR effect. Additionally, polymeric nanocarriers may be prepared from both natural polymers such as albumin [48], hyaluronic acid [49] and chitosan [50], and synthetic polymers such as poly acrylamide (PAA), polylactic acid (PLA), poly glycolic acid (PGA), poly(lactide-co-glycolide) (PLGA) and polypeptide [51].
4.2.1. Synthetic polymer NPs 4.2.1.1. Biodegradable polymers NPs.Biodegradable polymers are biocompatible polymers including poly(orthoesters), poly(β-ami- no esters) (PbAE) as well as poly(α-hydroxy esters); the latter which contains poly(D, L-lactide) (PLA), poly(glycolide) (PGA) and poly(ε-caprolactone) (PCL) are the most extensively utilized polymers for the construction of micelles and NPs. Earlier reports on biodegradable polymeric NPs as delivery systems of PSs showed that they had a greater photodynamic efficiency, reduced photosensitivity, improved pharmacokinetic properties and targeted ability through modification of their surface with a variety of biomolecules [29]. These previous studies about the biodegradable polymers of nanoconstructs, photocytotoxicity, biodistribution and PDT efficiency have been reviewed by Bechet [52] and Chatterjee [53] in 2008. Furthermore, due to rapid opsonisation and removal from the systemic circulation by the macrophage cells of the MPS with regard of the biodegradable polymers, systemic use of such synthetic polymeric NPs has been limited. To tackle this problem, NPs are often further modified by PEG to endow stealth properties [54]. PEGylated biodegradable polymers NPs have been exhibited to have enhanced circulation time and remain in the blood compared to bare biodegradable polymers NPs [55]. For example, PEGylated PCL NPs loaded with both docetaxel (DTX) and zinc phthalocyanine (ZnPc) employing the melting/sonication method could keep stability in the presence of serum proteins and plasma (Fig. 3A) [56]. The micelles exhibited efficient 1O2 generation and improved cytotoxicity against HeLa cells due to a synergistic effect contributed by the anti-cancer drug DTX. Hence, this design demonstrated the potential of synergistic treatment in cancer therapy using PDT and chemotherapy.

|
Download:
|
| Figure 3. (A) Schematic illustration of the NPs formed from PEO-b-PCL or PCL-b-PEO-b-PCL for synergistic treatment in cancer treatment using PDT and chemotherapy. Reproduced with permission from ref. [56]. Copyright 2013 Elsevier. (B) Synthetic procedure for the multifunctional polymer nanomedicines. Reproduced with permission from ref. [57]. Copyright 2012 American Chemical Society. | |
Multifunctional biodegradable polymeric NPs based delivery systems gained more attentions, and various studies have been reported in the application of polymeric NPs in carrying both diagnostic and therapeutic payload. Wang et al. developed multifunctional nanocarriers, based on amine-functionalized biodegradable polyacrylamide NPs, for cancer theranostics. The structural design involved adding primary amino groups and biodegradable cross-linkers during the NP polymerization, while incorporating photodynamic and fluorescent imaging agents into the NP matrix,and conjugating PEG and tumor-targeting ligands on to the surface of the NPs (Fig. 3B) [57]. The multifunctional NPs containing both fluorophores,PS,and tumor-targeting moieties, emitted bright fluorescence and also generated 1O2 under light irradiation,leading to an irreversible but selective destruction of the cancer cells. Similarly,a multifunctional nanostructure (ICGFA-PPD) via the self-assembly of indocyanine green (ICG) and FA decorated PEI-PEG-gadoteric acid (Gd-DOTA) (FA-PPD) with welldefined structural characteristics and physical properties was reported by Tan et al. [58]. The resulting nanostructures not only provided the performance of tumor targeting,MRI,and optical imaging,but more importantly,enabled PDT for in vivo tumor treatment. Yet another process for triple modal fluorescence, MR, and photoacoustic (PA) imaging-guided associated with photothermal (PTT) and PDT,was recently reported by Gong et al. using a nanomicellar system [59]. The multifunctional polymeric nanomicelle system was successfully prepared through grafting a PS Ce6 to an amphiphilic polymer composed of poly(maleic anhy- dride-alt-1-octadecene) and PEG chains, at the same time serving as a chelating agent for Gd3+, together with a near-infrared (NIR) dye, IR825. Additionally, triple modal fluorescence, MR and PA were conducted in a mouse model, revealing the efficient and homogenous uptake of NPs in the tumor. The combined PTT and PDT was then performed, achieving a synergistic anti-tumor effect both in vitro and in vivo experiments. Therefore, this work presented a polymer based theranostic platform with a great potential in multimodal imaging and combination therapy of cancer. Taratula and Alani reported the development and use of biodegradable-biocompatible silicon naphthalocyanine (SiNc)- loaded polymeric NPs for image-guided combinatorial phototherapy with dual PTT and PDT [60]. This theranostic nanoplatform combined SiNc as a NIR fluorescence imaging and phototherapeutic agent with PEG-b-PCL as the biodegradable SiNc carrier. SiNc-polymeric NPs demonstrated excellent photostability and efficient generation of NIR fluorescence, ROS, and hyperthermia under NIR light illumination needed for tumor imaging and cancer eradication via combined PDT and PTT mechanisms. Thus, this strategy provided cancer imaging with a single-agent theranostic nanoplatform and subsequent phototherapeutic treatment with great potential for clinical translation.
4.2.1.2. Responsive polymer NPs.Through PDT's localized property, the ability to activate a PS in response to a specific stimulus has been applied in the field of tumor treatments. This strategy maintains the PS in a quenched state during its systemic circulation, and then activates the PS when it accumulates in the diseased tissue with a suitable wavelength of light. This approach could decrease the risk for off-target activation and skin photosensitivity. The mechanism that maintains the "off" state in the PS is performed through either a static quenching or a FRET quenching.
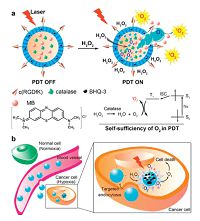
Kim et al. developed a bioreducible biarmed methoxy poly(ethylene glycol)-(pheophorbide a)2 (mPEG-(ss-PhA)2) conjugate for cancer-cell specific PDT [61]. Here, two PhA moieties were chemically conjugated to one mPEG molecule via disulfide bonds. The stable core-shell spherical NPs constructed by the amphiphilic polymer under aqueous conditions showed intramolecular and intermolecular self-quenching effects that enabled the NPs to remain photoinactive in a physiological buffer. This property would minimize deleterious effect because of any 1O2 generation during its systemic circulation. However, when the NPs entered the cytosol of the tumor cells and exposed to a reductive environment, the PhA moieties in a photoactive form were rapid released due to the cleavage of the disulfide bonds in response to intracellular reductive conditions. In vitro cell experiments revealed that the NPs had a significant phototoxicity in cancer cells,and the cytotoxicity was lower than that of free PhA. These NPs could offer a potential approach of controllable 1O2 generation within cancer cells,which could result in more effective PDT with minimal phototoxicity in normal tissue,and then increased efficacy for PDT. Recently,Guo et al. reported a cell specific,H2O2-activatable,and O2-evolving PDT nanoparticle (HAOP NP) for highly selective and efficient cancer treatment (Fig. 4) [62]. When the HAOP NP was selectively taken up by αvβ3 integrin-rich tumor cells,the intracellular H2O2 penetrated the shell into the core and was catalyzed by catalase to generate O2,resulting in the shell rupture and release of PSs. The released PSs can generate cytotoxic 1O2 under illumination and the presence of O2 to kill cancer cells.
|
Download:
|
| Figure 4. Schematic illustration of (a) mechanism of H2O2-controllable release of PS and O2 to implement PDT and (b) HAOP NP for selective and efficient PDT against hypoxic tumor cell. Reproduced with permission from ref. [62]. Copyright 2014 American Chemical Society. | |
Acid-sensitive systems based on the difference between the normal and tumor physiology could be utilized. Na et al. utilized the pH differences between the normal (pH remains constant at 7.4) and tumor (pH below 7.2) tissue, to develop tumor pH- sensitive magnetic nanogrenades (termed PMNs) composed of self-assembled iron oxide NPs and pH-responsive ligands [63]. These PMNs can readily target tumors through surface- charge switching triggered by the acidic tumor microenvironment and further disassembled into a highly active state in acidic subcellular compartments that "turns on" MR contrast, fluorescence and photodynamic therapeutic activity in a site-specific manner. Recently, Han et al. developed an amphiphilic chimeric peptide to realize sequential acidity-responsive tumor-targeted transport of PS and in situ PDT in nuclei. Similarly, Li etal. reported acid-switchable multifunctional micelles for combinational photo/ chemotherapy of the drug-resistant tumor [64]. The micelles were prepared from a pH-responsive diblock copolymer, a PS, and a polymeric prodrug of doxorubicin. The micelles exhibited silent fluorescence, MR, and PDT activity during systemic circulation, which can be dramatically activated upon cellular uptake and intracellular dissociation in cancer cells. Upon NIR laser irradiation, the activated micelles generated ROS and hyperthermia effect for combinational photo/chemotherapy of drug-resistant tumor. Meanwhile, the hybrid micelles displayed good potential for fluorescence, MR, and PA triple-modal tumor imaging. The versatile micelles reported in this study might provide a novel insight for the combination of drug resistant tumors with nanoparticle drugs.
Enzymes are catalytic, diverse, and central to all facets of cellular function, therefore, they are excellent targets for PSs [65]. Since enzyme overexpression is correlated with specific diseases in many occasions, PS activation can be confined to the location of the active enzyme target, while in the blood not expressing the enzyme, the PS maintains inactive. Tung etal. firstly reported a new approach to selective PDT via conjugating numerous Ce6 molecules onto a polylysine backbone, which were sensitive to tumor-associated proteases [66]. The PS molecules were held in a close geometry for efficient self-quenching, thus prohibiting the generation of 1O2. In the presence of tumorassociated enzymes, the peptide linkages of the polylysine backbone were cleaved, and the disabled probes became highly phototoxic and fluorescent. Recently, Zhang et al. designed and synthesized a matrix metalloproteinase-2 (MMP-2) responsive ratiometric biosensor for aggregation-induced emission (AIE)- guided precise PDT [67]. This ratiometric biosensor successfully distinguished the expression degree of MMP-2 among different cell lines in spite of the biosensor concentration based on the fluorescence intensity ratio between tetraphenyl ethylene (TPE) and protoporphyrin IX (PpIX). More importantly, nanosized particles could effectively accumulate in the tumor tissue via EPR effect, and MMP-2 responsive dual fluorescence imaging of between TPE and PpIX showed the tumor tissue accurately, which guided the PDT in the tumor region, realizing improved therapeutic efficacy with minimal side effects. Subsequently, they also developed a conjugate of matrix metalloproteinases-2 (MMP- 2) -sensitive activable cell-penetrating peptide (R9GPLGLAGE8, ACPP) with PpIX for tumor-targeting PDT [68]. In normal tissue, the cell-penetrating function of polycationic CPP (R9) would be blocked by a polyanionic peptide (E8) through intramolecular electrostatic attraction. Once the oligopeptide linker (GPLGLAG) between the CPP and the polyanionic peptide exposed to MMP-2 at the tumor tissue, it could generate proteolysis, leading to dissociate the inhibitory polyanions and release CPP-PpIX for PDT. This ACPP-PpIX conjugate delivery system would be a promising strategy for tumor diagnostic imaging and targeting therapies.
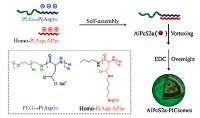
Recently, light-responsive systems have attracted much attention for phytochrome delivery. Liu et al. designed a polymeric gene delivery vector, which contained a PS with AIE properties and oligoethylenimine (OEI) conjugated based on an aminoacrylate (AA) linker that can be cleaved by ROS generated through PS under special irradiation [69]. In aqueous solution, the polymer could self-assemble into bright red fluorescent NPs, which can efficiently bind to DNA through electrostatic interaction for gene delivery. Upon light illumination, the gene delivery vector could simultaneously lead to endo/lysosomal escape and DNA unpacking, which can efficiently deliver DNA to the cytosol of cells with low cytotoxicity. The smart polymer represented the first successful gene delivery systems to concurrently handle both challenges with a single light excitation process. Additionally, Kataoka and coworkers demonstrated photoinduced release of aluminum phtha- locyanine disulfonate (AlPcS2a) from the polymer vesicle termed "PICsomes". The PICsomes prepared by a pair of oppositely charged PEG-based block aniomer and homocatiomer (Fig. 5) [70]. The PICsomes can be applied to encapsulate AlPcS2a and photoinduced release of the photoactive agents for intracellular drug delivery. The AlPcS2a quick release was induced by photoirradiation possibly due to the photochemical damage of the PIC membranes. Simultaneously, the released AlPcS2a photochemically damaged the integrity of the lysosomal membranes, resulting in the translocation of AlPcS2a and PICsomes themselves to the cytoplasm. This approach showed a stronger photocytotoxicity compared with free AlPcS2a alone. Thus, the AlPcS2a-PICsomes have promising feasibility for the PDT or the photoinduced cytoplasmic delivery of therapeutic molecules.
|
Download:
|
| Figure 5. Schematic illustration of AlPcS2a-PICsomes. Reproduced with permission from ref. [70]. Copyright 2014 American Chemical Society. | |
4.2.2. Natural polymeric NPs
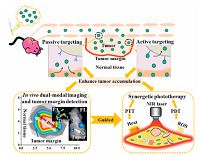
Natural polymers are attractive components in formulating carriers of PSs because of their advantages of their low cost, abundance, water solubility, biodegradability, and biocompatibility [71]. Albumin is the most abundant plasma protein and has been applied extensively for the construction of nanocapsules due to its availability, biodegradability, nontoxicity, non- immunogenicity, hydrophilicity, and ease of preparation [72]. Human serum albumin (HSA) based NPs have attracted great interest as theranostic drug carrier due to its high binding capacity for various drugs. Moreover, the endogenous albumin can select appropriate route for delivering large amounts of chemotherapeutic drugs to the tumor tissue [73]. Wacker et al. prepared albumin-based NPs for encapsulating the PS via an absorptive drug loading technique [74]. After incubation with Jurkat cells, PS-loaded NPs efficiently generated 1O2, which suggested that the PSs were released from NPs upon cell uptake. Additionally, Cai et al. developed a programmed assembly strategy for the preparation of HSA-indocyanine green (ICG) NPs (HSA-ICG NPs) by intermolecular disulfide conjugations (Fig. 6) [75]. The HSA-ICG NPs enhanced delivery of ICG into cancer cells. The tumor, tumor margin and normal tissue could be clearly detected using ICG-based in vivo FL and PA dual-modal imaging and spectrum-resolved technology. Simultaneously, PDT/PTT treatments significantly improved the anticancer effect, leading to superior tumor eradication without any regrowth. The results revealed that HSA-ICG NPs prepared by programmed assembly as smart theranostic nanoplatforms were highly potential for imaging-guided cancer phototherapy with PDT/ PTT synergistic effects. Furthermore, a self-assembled albumin- based nanoprobe was successfully fabricated by Liu et al. for realtime pH sensing of tumors under noninvasive ratiometric PA imaging [76]. Ratiometric PA imaging in combination with quantitative analysis using albumin-based nanoprobe could be used to detect the tumor microenvironment pH. Therefore, this work could offer a great tool for in vivo noninvasive, real-time, quantitative pH imaging, particularly useful for studying of tumor microenvironment.
|
Download:
|
| Figure 6. Schematic illustration of HSA-ICG NPs for in vivo dual-modal imaging,tumor margin detection,and simultaneous PDT/PTT treatments. Uptake of HSA-ICG NPs is presumably mediated by EPR effect (passive targeting) and the gp60 transcytosis pathway (active targeting) and subsequent binding to SPARC (secreted protein acidic and rich in cysteine,SPARC) in the tumor cells. The tumor,tumor margin and normal tissue could be detected using in vivo NIR and PA dual-modal imaging and spectrum-resolved technology. Upon the single NIR laser irradiation,the HSA-ICG NPs can simultaneously convert the absorbed light energy to ROS and heat for synergistic PDT/PTT treatments. Reproduced with permission from ref. [75]. Copyright 2014 American Chemical Society. | |
Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan that occurs naturally in high concentrations in various soft connective tissues such as synovial fluid, vitreous humor, skin, and the umbilical cord [76]. HA was first isolated in 1934 from bovine vitreous humor by Meyer and Palmer [77, 78]. HA-based NPs have various advantages including tumor targeting ability, selectivity release, and biocompatibility. For example, Yoon etal. designed the use of tumor-targeting hyaluronic acid NPs (HANPs) to deliver hydrophobic Ce6 for simultaneous photodynamic imaging and therapy [79]. In this investigation, aminated 5-b-cholanic acid, black hole quencher 3, and PEG were conjugated to HA polymers and Ce6 was loaded by dialysis. The NPs were internalized by cells due to CD44 receptor binding. Upon internalization, Ce6-HANPs were rapid degraded by hyaluronidase, enabling intracellular release of Ce6 at the tumor tissue and further improving the efficacy of PDT. After intravenous injection into tumor-bearing mice, the Ce6-loaded HA NPs can efficiently accumulate in the tumor tissue. Upon laser irradiation, the released Ce6 can generate fluorescence and 1O2 inside tumor cells and effectively suppress tumor growth. Recently, Na et al. demonstrated a tumor-specific photoactivity-controllable NP photomedicine based on a combination of PS-biomacromolecule conjugates and polydopamine NPs (PD-NP) for an effective tumor therapy [80]. The PD-NP and the PS- HA all played a role as the quencher for PSs and a cancer targeting moiety, respectively. The synthesized PS-HA-shielded PD-NPs (PHPD-NPs) had a relatively narrow size distribution with uniform spherical shapes. In response to cancer-specific intracellular hyaluronidase, the PHPD-NPs exhibited an excellent 1O2 generation capacity for PDT. Furthermore, an efficient photothermal conversion ability for PTT was also shown in the PHPD-NPs system. These properties provided a superior therapeutic efficacy against cancer cells. In mice tumor model, the photoactive restorative effects of the PHPD-NPs were much higher in cancer microenvironments compared to that in the normal tissue.
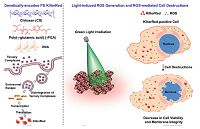
Chitosan is a polysaccharide derived from the partial deacetylation of chitin, primarily from crustacean and insect shells. It consists of repeating units of glucosamine and N-acetyl-glucos- amine. Although chitosan is insoluble at neutral pH, it is soluble and positively charged at acidic pH [81]. Several investigations have reported the application of amphiphilic chitosan-based NPs as carriers of hydrophobic anticancer drugs in their inner cores [82]. For PDT, beside physical encapsulation, NPs can carry PSs by direct conjugation of the PS with specific groups of the chitosan. In this case, drug-conjugated NPs are able to manipulate therapeutic agents at the molecular level. Oh etal. constructed a cancer-specific PS nanocarrier PhA-ss-CNPs by conjugating PhA with glycol chitosan (GC) through reducible disulfide bonds [83]. In solution, PhA-ss-CNPs can self-assemble into stable nanostructures with quenched photoactivity. The PhA-ss-GC conjugates can self- assemble in aqueous condition to form core-shell structured NPs (PhA-ss-CNPs) with quenched photoactivity. After they were taken up by cancer cells, NPs immediately disassemble by reductive cleavage of the disulfide linkers and undergo a dequenching process. Neither free CNPs nor free PhA exhibited cytotoxicity in the absence of irradiation. However, after 20 min of irradiation, PhA-ss-CNPs and free PhA treatment resulted in 45% increase in cytotoxicity compared to non-irradiated samples. PhA- ss-CNP also exhibited prolonged blood circulation when compared to free PhA, allowing tumor targeting by the EPR effect. Enhanced tumor accumulation increased the efficacy of PhA-ss-CNP against HT29 tumor-bearing. These results showed that PhA-ss-CNP was an attractive system for cancer PDT. Sung et al. investigated complexes of CS and poly(g-glutamic acid) (gPGA) to deliver the genetic use of pKillerRed-mem, to intracellularly express a membrane-targeted KillerRed protein that can be used as a potential PS for PDT (Fig. 7) [84]. Following transfection with CS/ pKillerRed/gPGA complexes, a red fluorescence protein of KillerRed was clearly seen at the cellular membranes. When exposed to green light irradiation, the KillerRed-positive cells produced an excessive amount of ROS in a time-dependent manner. Data from viability assays indicated that ROS had an important role in mediating KillerRed-induced cytotoxicity, apoptosis, and antiproliferation, suggesting that KillerRed could be used as an intrinsically generated PS for PDT treatments. Notably, the phototoxic reaction of KillerRed toward cells gradually became negligible over time, presumably because of its intracellular degradability. These experimental results demonstrated that this genetically encoded KillerRed would be potential for PDT-induced destruction of diseased cells.
|
Download:
|
| Figure 7. Schematic illustrations showing the working mechanism of a genetically encoded KillerRed protein as an intrinsically generated PS for PDT. Reproduced with permission from ref. [84]. Copyright 2014 Elsevier. | |
5. Conclusion and future perspectives
In this review, we have summarized the fascinating development of various organic NPs and their application in PDT. PDT is undoubtedly a highly effective therapeutic alternative for the treatment of cancers due to improvements in light generation and delivery technologies. However, due to limitations of PSs, their full potential has not been realized regarding the treatment of cancers. Since the majority of first and second generation PSs have drawbacks such as poor solubility in the physiological environments, adverse pharmacokinetics and poor tumor selectivity, NP formulations provide a method to overcome the mentioned limitations. In addition, surface modification with targeting ligands could further improve the selective accumulation of PSs loaded NPs at the target site. However, despite the satisfactory results reported in the past few years in this field, there are still many challenges ahead toward further clinical applications of those functional nanomaterials in PDT of cancer. One of the most important issues is the potential long term safety concerns of the nanomaterials after administration. Another major challenge in PDT of cancer is the limited light penetration depth. Thirdly, imaging guided therapy becomes extremely meaningful and important in PDT to improve the efficacy of treatment and reduce the side effect of normal tissue. Lastly, each therapeutic approach could have its own advantages and limitations. Therefore, explorations with respect to the multifunctional therapy of cancers by integrating PDT methods with other therapies to achieve the synergistic cancer killing effect may bring great opportunities to the new generation of cancer therapy. Nevertheless, it is believed that PDT based on functional organic NPs, due to their unique advantages such as minimal side effects and high efficacies, would play increasingly important roles in the fight against cancers.
Acknowledgments This work was financially supported by the National Natural Science Foundation of China (No. 21574039), Research Innovation Program of SMEC (No. 14ZZ065) and Shanghai Pujiang Program (No. 14PJ1402600).| [1] | M. Ferrari. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5 (2005) 161–171. DOI:10.1038/nrc1566 |
| [2] | T.J. Dougherty, G.B. Grindey, R. Fiel, K.R. Weishaupt, D.G. Boyle. photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J. Natl. Cancer Inst. 55 (1975) 115–121. |
| [3] | W.M. Sharman, C.M. Allen, J.E. van Lier. Role of activated oxygen species in photodynamic therapy, in: Methods in Enzymology. Academic Press, New York pp. (2000) 367–400. |
| [4] | D.E.J.G.J. Dolmans, D. Fukumura, R.K. Jain. Photodynamic therapy for cancer. Nat. Rev. Cancer 3 (2003) 380–387. DOI:10.1038/nrc1071 |
| [5] | P. Mroz, A. Yaroslavsky, G.B. Kharkwal, M.R. Hamblin. Cell death pathways in photodynamic therapy of cancer. Cancers 3 (2011) 2516–2539. DOI:10.3390/cancers3022516 |
| [6] | P. Baluk, H. Hashizume, D.M. McDonald. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 15 (2005) 102–111. DOI:10.1016/j.gde.2004.12.005 |
| [7] | C. Abels. Targeting of the vascular system of solid tumours by photodynamic therapy (PDT). Photochem. Photobiol. Sci. 3 (2004) 765–771. DOI:10.1039/b314241h |
| [8] | R.R. Allison, K. Moghissi. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 46 (2013) 24–29. DOI:10.5946/ce.2013.46.1.24 |
| [9] | M. Niedre, M.S. Patterson, B.C. Wilson. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 75 (2002) 382–391. DOI:10.1562/0031-8655(2002)0750382DNILDO2.0.CO2 |
| [10] | J. Moan, K. Berg. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 53 (1991) 549–553. DOI:10.1111/php.1991.53.issue-4 |
| [11] | B. Green, A.R.M. Cobb, C. Hopper. Photodynamic therapy in the management of lesions of the head and neck. Br. J. Oral Maxillofac. Surg. 51 (2013) 283–287. DOI:10.1016/j.bjoms.2012.11.011 |
| [12] | R. Allison, K. Moghissi, G. Downie, K. Dixon. Photodynamic therapy (PDT) for lung cancer. Photodiagnosis Photodyn. Ther. 8 (2011) 231–239. DOI:10.1016/j.pdpdt.2011.03.342 |
| [13] | M.B. Ericson, A.M. Wennberg, O. Larkö. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther. Clin. Risk Manag. 4 (2008) 1–9. |
| [14] | C.M.B. Carvalho, J.P.C. Tomé, M.A.F. Faustino, et al. Antimicrobial photodynamic activity of porphyrin derivatives: potential application on medical and water disinfection. J. Porphyrins Phthalocyanines 13 (2009) 574–577. DOI:10.1142/S1088424609000528 |
| [15] | S.S. Lucky, K.C. Soo, Y. Zhang. Nanoparticles in photodynamic therapy. Chem. Rev. 115 (2015) 1990–2042. DOI:10.1021/cr5004198 |
| [16] | K.K. Ng, G. Zheng. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem. Rev. 115 (2015) 11012–11042. DOI:10.1021/acs.chemrev.5b00140 |
| [17] | W.M. Sharman, J.E. van Lier, C.M. Allen. Targeted photodynamic therapy via receptor mediated delivery systems. Adv. Drug Deliv. Rev. 56 (2004) 53–76. DOI:10.1016/j.addr.2003.08.015 |
| [18] | D. Kozlowska, P. Foran, P. MacMahon, et al. Molecular and magnetic resonance imaging: the value of immunoliposomes. Adv. Drug Deliv. Rev. 61 (2009) 1402–1411. DOI:10.1016/j.addr.2009.09.003 |
| [19] | M. Ethirajan, Y.H. Chen, P. Joshi, R.K. Pandey. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 40 (2011) 340–362. DOI:10.1039/B915149B |
| [20] | T.A. Debele, S. Peng, H.C. Tsai. Drug carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 16 (2015) 22094–22136. DOI:10.3390/ijms160922094 |
| [21] | A.B. Ormond, H.S. Freeman. Dye sensitizers for photodynamic therapy. Materials 6 (2013) 817–840. DOI:10.3390/ma6030817 |
| [22] | S.S. Stylli, A.H. Kaye, L. MacGregor, M. Howes, P. Rajendra. Photodynamic therapy of high grade glioma-long term survival. J. Clin. Neurosci. 12 (2005) 389–398. DOI:10.1016/j.jocn.2005.01.006 |
| [23] | C. Staneloudi, K.A. Smith, R. Hudson, et al. Development and characterization of novel photosensitizer: scFv conjugates for use in photodynamic therapy ofcancer. Immunology 120 (2007) 512–517. DOI:10.1111/j.1365-2567.2006.02522.x |
| [24] | R.R. Allison. Photodynamic therapy: oncologic horizons. Future Oncol. 10 (2014) 123–124. DOI:10.2217/fon.13.176 |
| [25] | Z. Huang, H.P. Xu, A.D. Meyers, et al. Photodynamic therapy for treatment of solid tumors-potential and technical challenges. Technol. Cancer Res. Treat. 7 (2008) 309–320. DOI:10.1177/153303460800700405 |
| [26] | A. Prokop, J.M. Davidson. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 97 (2008) 3518–3590. DOI:10.1002/jps.21270 |
| [27] | C. Luo, J. Sun, B.J. Sun, Z.G. He. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharm. Sci. 35 (2014) 556–566. DOI:10.1016/j.tips.2014.09.008 |
| [28] | E. Paszko, C. Ehrhardt, M.O. Senge, D.P. Kelleher, J.V. Reynolds. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 8 (2011) 14–29. DOI:10.1016/j.pdpdt.2010.12.001 |
| [29] | Y.N. Konan, R. Gurny, E. Allémann. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B: Biol. 66 (2002) 89–106. DOI:10.1016/S1011-1344(01)00267-6 |
| [30] | R.R. Sawant, V.P. Torchilin. Liposomes as ‘smart’ pharmaceutical nanocarriers. Soft Matter 6 (2010) 4026–4044. DOI:10.1039/b923535n |
| [31] | J.F. Lovell, C.S. Jin, E. Huynh, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10 (2011) 324–332. DOI:10.1038/nmat2986 |
| [32] | E. Huynh, G. Zheng. Porphysome nanotechnology: a paradigm shift in lipid-based supramolecular structures. Nano Today 9 (2014) 212–222. DOI:10.1016/j.nantod.2014.04.012 |
| [33] | A.S.L. Derycke, P.A.M. de Witte. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 56 (2004) 17–30. DOI:10.1016/j.addr.2003.07.014 |
| [34] | D.D. Lasic, F.J. Martin, A. Gabizon, S.K. Huang, D. Papahadjopoulos. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim. Biophys. Acta 1070 (1991) 187–192. DOI:10.1016/0005-2736(91)90162-2 |
| [35] | Y. Sadzuka, F. Iwasaki, I. Sugiyama, et al. Phototoxicity of coproporphyrin as a novel photodynamic therapy was enhanced by liposomalization. Toxicol. Lett. 182 (2008) 110–114. DOI:10.1016/j.toxlet.2008.09.002 |
| [36] | R. Rahmanzadeh, P. Rai, J.P. Celli, et al. Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer. Cancer Res. 70 (2010) 9234–9242. DOI:10.1158/0008-5472.CAN-10-1190 |
| [37] | N. Oku, T. Ishii. Chapter 16: Antiangiogenic photodynamic therapy with targeted liposomes, in: Methods in Enzymology. Academic Press, New York pp. (2009) 313–330. |
| [38] | C.S. Jin, L.Y. Cui, F. Wang, J. Chen, G. Zheng. Targeting-triggered porphysome nanostructure disruption for activatable photodynamic therapy. Adv. Healthc. Mater. 3 (2014) 1240–1249. DOI:10.1002/adhm.v3.8 |
| [39] | C.S. Jin, G. Zheng. Liposomal nanostructures for photosensitizer delivery. Lasers Surg. Med. 43 (2011) 734–748. DOI:10.1002/lsm.v43.7 |
| [40] | P. Skupin-Mrugalska, J. Piskorz, T. Goslinski, et al. Current status of liposomal porphyrinoid photosensitizers. Drug Discov. Today 18 (2013) 776–784. DOI:10.1016/j.drudis.2013.04.003 |
| [41] | A. Yavlovich, B. Smith, K. Gupta, R. Blumenthal, A. Puri. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol. Membr. Biol. 27 (2010) 364–381. DOI:10.3109/09687688.2010.507788 |
| [42] | S. Simões, J.N. Moreira, C. Fonseca, N. Düzgüneş, M.C. Pedroso de Lima. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 56 (2004) 947–965. DOI:10.1016/j.addr.2003.10.038 |
| [43] | B. Spring, Z.M. Mai, P. Rai, S. Chang, T. Hasan, Theranostic nanocells for simultaneous imaging and photodynamic therapy of pancreatic cancer, in: Proceedings of SPIE 7551, Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XIX, SPIE, San Francisco, CA, 2010, pp. 755104-755111. |
| [44] | X.L. Liang, X.D. Li, X.L. Yue, Z.F. Dai. Conjugation of porphyrin to nanohybrid cerasomes for photodynamic diagnosis and therapy of cancer. Angew. Chem. Int. Ed. 50 (2011) 11622–11627. DOI:10.1002/anie.201103557 |
| [45] | O. Salata. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2 (2004) 3. DOI:10.1186/1477-3155-2-3 |
| [46] | Y.M. Zhou, X.L. Liang, Z.F. Dai. Porphyrin-loaded nanoparticles for cancer theranostics. Nanoscale 8 (2016) 12394–12405. DOI:10.1039/C5NR07849K |
| [47] | S.Y. Wang, W.Z. Fan, G. Kim, et al. Novel methods to incorporate photosensitizers into nanocarriers for cancer treatment by photodynamic therapy. Lasers Surg. Med. 43 (2011) 686–695. DOI:10.1002/lsm.v43.7 |
| [48] | A.O. Elzoghby, W.M. Samy, N.A. Elgindy. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 157 (2012) 168–182. DOI:10.1016/j.jconrel.2011.07.031 |
| [49] | K.Y. Choi, H. Chung, K.H. Min, et al. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 31 (2010) 106–114. DOI:10.1016/j.biomaterials.2009.09.030 |
| [50] | S.M. Abdelghany, D. Schmid, J. Deacon, et al. Enhanced antitumor activity of the photosensitizer meso-tetra(N-methyl-4-pyridyl) porphine tetra tosylate through encapsulation in antibody-targeted chitosan/alginate nanoparticles. Biomacromolecules 14 (2013) 302–310. DOI:10.1021/bm301858a |
| [51] | J. Panyam, V. Labhasetwar. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55 (2003) 329–347. DOI:10.1016/S0169-409X(02)00228-4 |
| [52] | D. Bechet, P. Couleaud, C. Frochot, et al. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 26 (2008) 612–621. DOI:10.1016/j.tibtech.2008.07.007 |
| [53] | D.K. Chatterjee, L.S. Fong, Y. Zhang. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv. Drug Deliv. Rev. 60 (2008) 1627–1637. DOI:10.1016/j.addr.2008.08.003 |
| [54] | J.R. McCarthy, J.M. Perez, C. Brückner, R. Weissleder. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 5 (2005) 2552–2556. DOI:10.1021/nl0519229 |
| [55] | A. Beletsi, Z. Panagi, K. Avgoustakis. Biodistribution properties of nanoparticles based on mixtures of PLGA with PLGA-PEG diblock copolymers. Int. J. Pharm. 298 (2005) 233–241. DOI:10.1016/j.ijpharm.2005.03.024 |
| [56] | C. Conte, F. Ungaro, G. Maglio, et al. Biodegradable core-shell nanoassemblies for the delivery of docetaxel and Zn(II)-phthalocyanine inspired by combination therapy for cancer. J. Control. Release 167 (2013) 40–52. DOI:10.1016/j.jconrel.2012.12.026 |
| [57] | S.Y. Wang, G. Kim, Y.E.K. Lee, et al. Multifunctional biodegradable polyacrylamide nanocarriers for cancer theranostics-a "see and treat" strategy. ACS Nano 6 (2012) 6843–6851. DOI:10.1021/nn301633m |
| [58] | H. Wu, H.H. Wang, H. Liao, et al. Multifunctional nanostructures for tumortargeted molecular imaging and photodynamic therapy. Adv. Healthc. Mater. 5 (2016) 311–318. DOI:10.1002/adhm.v5.3 |
| [59] | H. Gong, Z.L. Dong, Y.M. Liu, et al. Engineering of multifunctional nano-micelles for combined photothermal and photodynamic therapy under the guidance of multimodal imaging. Adv. Funct. Mater. 24 (2014) 6492–6502. DOI:10.1002/adfm.v24.41 |
| [60] | O. Taratula, B.S. Doddapaneni, C. Schumann, et al. Naphthalocyanine-based biodegradable polymeric nanoparticles for image-guided combinatorial phototherapy. Chem. Mater. 27 (2015) 6155–6165. DOI:10.1021/acs.chemmater.5b03128 |
| [61] | W.L. Kim, H. Cho, L. Li, H.C. Kang, K.M. Huh. Biarmed poly(ethylene glycol)-(pheophorbide a)2 conjugate as a bioactivatable delivery carrier for photodynamic therapy. Biomacromolecules 15 (2014) 2224–2234. DOI:10.1021/bm5003619 |
| [62] | H.C. Chen, J.W. Tian, W.J. He, Z.J. Guo. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 137 (2015) 1539–1547. DOI:10.1021/ja511420n |
| [63] | D.S. Ling, W. Park, S.J. Park, et al. Multifunctional tumor pH-sensitive selfassembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J. Am. Chem. Soc. 136 (2014) 5647–5655. DOI:10.1021/ja4108287 |
| [64] | T.T. Wang, D.G. Wang, H.J. Yu, et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano 10 (2016) 3496–3508. DOI:10.1021/acsnano.5b07706 |
| [65] | S.I. Shoda, H. Uyama, J.I. Kadokawa, S. Kimura, S. Kobayashi. Enzymes as green catalystsforprecisionmacromolecularsynthesis. Chem.Rev. 116 (2016) 2307–2413. DOI:10.1021/acs.chemrev.5b00472 |
| [66] | Y. Choi, R. Weissleder, C.H. Tung. Selective antitumor effect of novel proteasemediated photodynamic agent. Cancer Res. 66 (2006) 7225–7229. DOI:10.1158/0008-5472.CAN-06-0448 |
| [67] | K. Han, S.B. Wang, Q. Lei, J.Y. Zhu, X.Z. Zhang. Ratiometric biosensor for aggregation-induced emission-guided precise photodynamic therapy. ACS Nano 9 (2015) 10268–10277. DOI:10.1021/acsnano.5b04243 |
| [68] | S.Y. Li, H. Cheng, W.X. Qiu, et al. Protease-activable cell-penetrating peptideprotoporphyrin conjugate for targeted photodynamic therapy in vivo. ACS Appl. Mater. Interfaces 7 (2015) 28319–28329. DOI:10.1021/acsami.5b08637 |
| [69] | Y.Y. Yuan, C.J. Zhang, B. Liu. A photoactivatable AIE polymer for light-controlled gene delivery: concurrent endo/lysosomal escape and DNA unpacking. Angew. Chem. Int. Ed. 54 (2015) 11419–11423. DOI:10.1002/anie.201503640 |
| [70] | H.B. Chen, L. Xiao, Y. Anraku, et al. Polyion complex vesicles for photoinduced intracellular delivery of amphiphilic photosensitizer. J. Am. Chem. Soc. 136 (2014) 157–163. DOI:10.1021/ja406992w |
| [71] | A.M. Bugaj. Targeted photodynamic therapy- a promising strategy of tumor treatment. Photochem. Photobiol. Sci. 10 (2011) 1097–1109. DOI:10.1039/c0pp00147c |
| [72] | L.S. Nair, C.T. Laurencin. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32 (2007) 762–798. DOI:10.1016/j.progpolymsci.2007.05.017 |
| [73] | W.J. Gradishar. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin. Pharmacother. 7 (2006) 1041–1053. DOI:10.1517/14656566.7.8.1041 |
| [74] | M. Wacker, K. Chen, A. Preuss, et al. Photosensitizer loaded HSA nanoparticles. I: Preparation and photophysical properties. Int. J. Pharm. 393 (2010) 254–263. DOI:10.1016/j.ijpharm.2010.04.022 |
| [75] | Z.H. Sheng, D.H. Hu, M.B. Zheng, et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano 8 (2014) 12310–12322. DOI:10.1021/nn5062386 |
| [76] | Q. Chen, X.D. Liu, J.W. Chen, et al. A self-assembled albumin-based nanoprobe for in vivo ratiometric photoacoustic pH imaging. Adv. Mater. 27 (2015) 6820–6827. DOI:10.1002/adma.201503194 |
| [77] | T.C. Laurent, J.R. Fraser. Hyaluronan. FASEB J. 6 (1992) 2397–2404. |
| [78] | J. Necas, L. Bartosikova, P. Brauner, J. Kolar. Hyaluronic acid (hyaluronan): a review. Vet. Med. 53 (2008) 397–411. |
| [79] | H.Y. Yoon, H. Koo, K.Y. Choi, et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials 33 (2012) 3980–3989. DOI:10.1016/j.biomaterials.2012.02.016 |
| [80] | J. Han, W. Park, S.J. Park, K. Na. Photosensitizer-conjugated hyaluronic acidshielded polydopamine nanoparticles for targeted photomediated tumor therapy. ACS Appl. Mater. Interfaces 8 (2016) 7739–7747. DOI:10.1021/acsami.6b01664 |
| [81] | R. Hejazi, M. Amiji. Chitosan-based gastrointestinal delivery systems. J. Control. Release 89 (2003) 151–165. DOI:10.1016/S0168-3659(03)00126-3 |
| [82] | J.H. Kim, Y.S. Kim, K. Park, et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials 29 (2008) 1920–1930. DOI:10.1016/j.biomaterials.2007.12.038 |
| [83] | I.H. Oh, H.S. Min, L. Li, et al. Cancer cell-specific photoactivity of pheophorbide aglycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 34 (2013) 6454–6463. DOI:10.1016/j.biomaterials.2013.05.017 |
| [84] | Z.X. Liao, Y.C. Li, H.M. Lu, H.W. Sung. A genetically-encoded KillerRed protein as an intrinsically generated photosensitizer for photodynamic therapy. Biomaterials 35 (2014) 500–508. DOI:10.1016/j.biomaterials.2013.09.075 |
 2016, Vol. 27
2016, Vol. 27