Conjugated microporous polymers (CMPs) are an emerging class of amorphous porous materials, which are constructed by aromatic compounds with conjugation linkages. The uniqueness of CMP is derived from the entire conjugation backbones and intrinsic micropore tunnels within a three-dimensional network. Compared with the other porous organic polymers such as covalent organic frameworks [1], polymers of intrinsic micropore [2], and hypercrosslinking polymers [3], the major advantage of CMP is its design, which has been accomplished by a variety of polycondensation reactions and building blocks. Since the first CMP was reported by Cooper et al. in 2007 [4], the development of CMPs has gained the significant progress, particularly on their synthesis and functionalization at the molecular level [5]. Also, modulation of morphology and size has been investigated to develop the CMP- type colloids, fibers, gels and films [6]. Thus CMPs promise the great potentials for more advanced fields with respect to the other porous materials. This short review specifically summarizes the recent progress of photo-functional CMPs on the studies of their performances and applications.
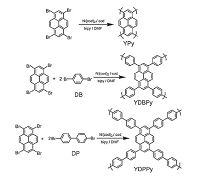
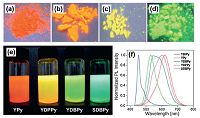
2. Solid-state photoluminescenceCMPs exhibit the extended π-conjugation system, wherein the building units are spatially segregated to suppress their π-π interaction. Thus incorporation of luminescent chromophores renders CMPs the remarkable fluorescent properties in the solid state. Weber and Thomas et al. reported the first example of a luminescent CMP derived from 9, 9'-spirobifluorene [7]. Afterwards, Cooper and co-workers synthesized a series of pyrene- based CMPs via the Yamamoto reaction (Fig. 1) [8]. Among them, the homopolymerized CMP from 1, 3, 6, 8-tetrabromopyrene showed the highest surface area up to 1508 m2/g and the red emission in the solid state. As the comonomer of 1, 4-dibromo- benzene or 4, 4'-dibromobiphenyl was polymerized with pyrene units, the emission colors ofthe obtained CMPs became orange and green, respectively (Fig. 2). The blue shift of fluorescence emission was possibly caused by the variation of pyrene content in the network. Also, the distribution of the pyrene units could influence the emission peak shapes.
|
Download:
|
| Figure 1. Synthesis of three pyrene-based CMPs via the Yamamoto reaction [8]. | |
|
Download:
|
| Figure 2. Fluorescence photographs of solid (a-d) and suspensions (e) ofYPy (a),YDPPy (b),YDBPy (c),and SDBPy (d) under 365-nm UV light,and their fluorescence spectra in the solid state (λex = 360nm) [8]. | |
Jiang et al. proposed the concept of “CMP effect” because the unique interlocked structure of CMP network could suppress the rotation of building blocks, promote the π-electronic conjugation, and intensify the luminescent activity irrespective of the solvent and material state. To prove it, they designed a light-emitting CMP based on tetraphenylethene (TPE) as knitting block, which is a typical chromophore with character of aggregation-induced emission in solid state [9]. Due to the interweaving of CMP backbones, the rotation of the phenyl groups on TPE units was largely limited, and the fluorescent enhancement was remarkable both in solvents and solid state. Subsequently, Jiang and coworkers synthesized a core-shell CMP consisting of a blue emissive polyphenylene CMP as the core and a yellow emissive polyte- traphenylethene CMP as the shell [10]. The light emission could be continuously tuned from blue to yellow by varying the core size and the shell thickness. Owing to the CMP effect, the luminescent activity was enhanced by 6-8 fold with respect to the linear analogues.
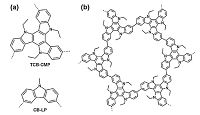
3. ChemosensorSince CMPs possess high surface areas, tunable light-emitting properties and flexible design routes, they have been proven to be effective chemosensors for use in the analytic field. In this system, sensing signals are activated by interaction of the specific sites with the guest compounds that are trapped in CMP networks. Jiang group reported the first example of molecular detection systems using the luminescent CMPs [11]. Polycondensation of carbazole- substituted monomers resulted in the CMP (Fig. 3) exhibiting the blue luminescence and the large surface area of as high as 1280 m2/ g. Moreover, its fluorescence could switch on/off depending on the arene vapors of the different electron properties. In comparison with the linear analogues, the CMP-type sensors displayed not only the enhanced detection sensitivity, but also the rapid response to arene molecules as exposed to their vapors. This was a consequence of the cooperative effect. The conjugated network facilitated the exciton migration over the skeleton, the large surface area enhanced the interfacial interaction on the adsorbed sites, and the micropore confined the diffusion of guest molecules.
|
Download:
|
| Figure 3. Schematic representation of (a) carbazole-based CMP (TCB-CMP) and linear analogue CB-LP,and (b) the network skeleton of TCB-CMP [11]. Reprinted with permission from Journal of the American Chemical Society. Copyright (2012) American Chemical Society. | |
Along this line, Jiang et al. adopted an electrochemical methodology to fabricate the thickness-controlled CMP films [12]. In this method, the monomer containing a 1, 3, 5-triphenyl- benzene core and three N-substituted carbazole groups at the periphery was electrochemically polymerized on electrodes. With increase of the CV cycles, the polymer network film grew thicker and could peel off the electrode to produce freestanding film with the thickness of above 50 nm. The surface area was estimated up to 1450 m2/g by Kr adsorption measurement at 77 K. The formed CMP film allowed for the sensitive fluorescence-on detection of the electron-rich arene vapor, and off for the electron-deficient one in 20s. Also, the fluorescence quenching was observed in response to oxidative metal ions, such as Fe3+, Co3+, and Ag+. This is due to the chemical oxidation of carbazole groups in the CMP film. Besides, the CMP film has been explored as the label-free biosensor for dopamine and hypochlorous acid on basis of the interaction of donor and acceptor.
The nanoscale CMPs have the improved dispersion in common solvents, and are susceptible of functionalization with versatile organic/inorganic components. Taking it into account, we prepared the poly(p-phenylene ethynylene)-based CMP (PPE-CMP) microspheres with the locked-in Fe3O4 nanocrystals by a toluene-inwater miniemulsion [13]. The hybrid nanomaterials had the combined properties that stemmed from the blue emissive conjugation skeleton, the large intrinsic surface area, and the catalytically active Fe3O4 nanocrystal. Through the cooperation of multiple functions, they could behave as fluorescence sensor for the detection of phenolic compounds in the presence of H2O2. The process involved that Fe3O4 catalyzed the oxidation of phenolic molecules to produce the reactive radicals, and the intermediates quickly decreased/quenched the fluorescence emission of PPE network.
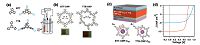
Apart from the fluorescence sensing, we designed a colorimetric detection system by using the dispersion of CMP microspheres (Fig. 4a) [14]. Metalloporphyrin-based CMP particles were synthesized in miniemulsion by the oxidative dimerization of terminal alkynes. The complexes of pyrrolidine and Zn(porphyrin) units within the CMP networks served as the active sites to allow for the visual detection of SO2 gas. Once the dispersion of CMP in chloroform was bubbled with SO2 gas, the solution turned red from the initial green color immediately (Fig. 4b). The discoloration mechanism could be explained that SO2 was competed with the complexes for pyrollidine and led to the release of Zn-porphyrin CMP that appeared red in its dispersion. The process was accompanied with the change in absorbance (Fig. 4c), providing the possibility of quantitative assay of SO2. We also demonstrated that the CMP microspheres could be employed to fabricate the coating on a paper for the gas-solid interfacial detection.

|
Download:
|
| Figure 4. (a) Discoloration mechanism of Zn(Porphyrin)-based CMP networks in the presence of SO2. (b and c) Photograph (b) and UV spectrum (c) of the dispersion of CMP microspheres in chloroform upon introduction of SO2 gas [14]. | |
4. Light harvesting
Exploration of artificial light-harvesting antenna is of significance in mimics of natural photosynthesis. Dendritic and hyperbranched polymers have been proven to be efficient energy-transferring systems, but they are plagued by the tedious and challenging synthesis [15]. In contrast, design and synthesis of CMPs are of course more facile and versatile, and intrinsic porous structure is their important advantage, by which acceptor molecules could be simply confined within micropores to establish an efficient donor-acceptor system. Jiang and co-workers, for the first time, investigated the light-harvesting property of poly(-phenylene)-based CMP (PP-CMP) (Fig. 5a) [16]. PP-CMP had the capability of blue fluorescence emitting, and enabled rapid transportation of charge carriers with the mobility of 0.04 cm2 V-1 s-1. When the energy-accepting molecule Coumarin 6 was filled in the micropores of PP-CMP, a donor-acceptor system was formed to undergo photon collection and energy transfer (Fig. 5b). Upon excitation of the PP-CMP with 363-nm light, the composite emitted strong green fluorescence from Coumarin 6, which was 21-fold as high as that of direct excitation of Coumarin 6, but no fluorescence from PP-CMP was observed. Thus an energy transduction from the light-harvesting PP-CMP skeleton to Coumarin 6 can be indicated. To be effective, increase of Coumarin 6 content could remarkably improve the energy transfer efficiency (Fig. 5c-e). It was calculated that almost 176 phenylene units of PP- CMP harvested the photons and channeled the excitation energy to one Coumarin 6. As for the composite with 2.9 mol% of Coumarin 6, its light-harvesting activity was roughly 27 times higher than that of 0.08 mol% system.

|
Download:
|
| Figure 5. (a) Structure representation of PP-CMP and Coumarin 6. (b) Illustration of light energy transfer from PP-CMP to Coumarin 6. (c) Fluorescence images of PP-CMP/ Coumarin 6 upon excitation at 363 nm in the solid state. (d) Fluorescence change of PP-CMP/Coumarin 6 by varying the Coumarin 6 contents upon excitation at 363 nm. (e) Plot of quantum efficiency of energy transfer from PP-CMP to Coumarin 6 [16]. Reprinted with permission from Journal of the American Chemical Society. Copyright (2010) American Chemical Society. | |
Successively, to estimate the cascade energy-transfer ability of CMP antennae, Jiang group synthesized a light-harvesting CMP film with the controlled thickness via the electrochemical method [17]. The CMP network comprised spirobifluorene blocks, and emitted blue fluorescence with the absolute quantum yield of 19%. To constitute a cascade energy transfer system, Coumarin 6 and Nile red were sequentially introduced into CMP films as the energy-accepting counterparts. Upon excitation of the CMP film, the energy transfer occurred from the CMP networks to Coumarin 6 and Coumarin 6 to Nile red. The emission colors of the composite films could be tuned from bright green to deep red by varying the loading content of Nile red within the CMP@Coumarin-6 system. The quantum yield of the red-emitting film (36%) was very close to that of monomeric Nile red (38%), indicating a complete energy transfer. Also, optimizing the contents of Coumarin 6 and Nile red within the CMP film could produce the white-light-emitting film. Studies on the mechanism of cascade energy transfer revealed that the efficiency and speed of energy transfer were both strongly dependent on the dye species and contents.
The dispersible and discrete CMP nanoparticles are another promising candidate to harvest the light energy through the conjugated skeletons, because their improved solution property facilitates the rational distribution of energy acceptor molecules. In our group, we synthesized the TPE-based hyperbranched particles by a routine miniemulsion and solvothermally treated them toward the luminescent CMP particles with the uniform morphology and grain size as well as the increased surface areas up to 1214 m2/g [18]. With the solvent diffusion, Nile red could be evenly distributed within the TPE-CMP network. It was reported that the energy-transfer efficiency was dependent on the surface areas of CMP microspheres, reaching as high as 90% for the high-surface-area CMP with 1.5 mol% of Nile red. Also, the rate constant of energy transfer was determined to be 1.9 × 108 s-1. Such rapid exciton diffusion over the CMP skeleton is unprecedented among CMPs and rarely found for other polymer antennas. In light of the dispersibility of CMPs in solution, the homogenous and transparent film was obtained by blending the CMP microspheres with poly(vinyl alcohol). As Phloxine B was doped in the film at concentrations of 0.1-0.4 mg/g, the film emission could be modulated over a wide range of the visible spectrum, including a pure white light.
5. Organic optoelectronicsAlthough CMPs show the great promise for the optoelectronic applications, the significant progress has not been achieved until the electropolymerization was applied to fabricate the CMP film on electrodes. Ma group firstly adopted the electrochemistry strategy to polymerize the carbazole-substituted tetrahedral monomers into a highly cross-linking network [19]. The thickness of CMP film could be precisely controlled by increasing the scan cycles and varying the oxidative potentials. The electrochemical doping was performed for the carbazole-based CMP films, resulting in a work function of 5.25 eV and conductivity of 46 S/m. The outstanding electric properties indicated that the CMP film was competent as an anode interlayer compared to PEDOT:PSS for polymer solar cells (PSCs) and polymer light-emitting diodes (PLEDs). The results proved that they could generate a power-conversion efficiency (PCE) of 7.56% in PSCs and a luminous efficiency of 20.7 cd/A in PLEDs.
Motivated by the early work, Jiang and co-workers developed the electropolymerization method for the synthesis of thiophene- based CMP film [20]. Two thiophene monomers were designed to electrochemically synthesize BTT-CMP and TTB-CMP films at the solution-electrode interface, respectively (Fig. 6a and b). The surface areas measured by Kr adsorption at 77 K were more than 1000 m2/g, and the pore size distribution was centered at 1.5 nm. By using the space-charge-limited current technique, hole mobility of BTT-CMP films was estimated up to 7.34 x 10-4 cm2 V-1 s-1, which is among the highest reported for π-conjugated polymers obtained with the same measurement. The energy acceptor C60 was loaded within the micropores of CMP films and the composite films were assembled into the solar cell as photoactive layers (Fig. 6c and d). The PCE was calculated up to 5.02% with the BTT- CMP/C60 composite. Subsequently, Jiang et al. designed the new CMP films for electrode interlayer, which were also electrochemically synthesized by using borane with three N-substituted carbazole groups at its periphery [21]. To adjust the work function of CMP film, the coordination of F- ion to the borane atom was conducted to obtain the fluorinated film, and in turn, it was electro-oxidized to transform the dimeric carbazole units into cations. The CMP film without any treatment had a low work function of 4.05 eV on ITO that had a work function of 4.78 eV, indicating that the electrode was endowed with the electron conduction property. While the CMP films were treated with F- ions and sequential oxidation, the work function greatly increased to 4.48 and 5.25 eV, respectively. The latter on ITO notably allowed for the injection or collection of holes in optoelectronic devices. On evaluation of the CMP films as photoactive layers, the low-work-function CMP film led to a PCE of 6.88% in the inverted PSC, and 7.93% for the high- work-function CMP film in the conventional PSC. Also, they exhibited the maximum luminous efficiency of 16.4 and 17.9 cd/A for the inverted PLED and the conventional PLED, respectively. The results again elucidated that the CMP films are superior to most of the conventional interlayer materials.
|
Download:
|
| Figure 6. (a) Structures of two thiophene-based monomers and illustration of a three-electrode electrochemical cell for the polymerization of monomers and the deposition of CMP films on ITO. (b) Elementary structures of the BTT-CMP and TTB-CMP and photographs of their films on ITO. (c) Device configuration of solar cells using BTT-CMP:C60 or TTB-CMP: C60 as the photoactive layer. (d) J-V curves of the solar cells with BTT-CMP:C60 (red curve) or TTB-CMP:C60 (blue curve) as the photoactive layer [20]. | |
6. Photocatalysis
Just like recently developed catalytic metal-organic frameworks, CMPs equally could act as a designable platform by incorporation of catalytic modules into CMP networks for the stable, recyclable and reusable solid catalysts. Photocatalysis is so attractive that synthetic chemists have devoted tremendous efforts over decades. CMPs also have been explored as photocatalysts due to their permanent porosity and the ability to tune the compositions and properties at the molecular level. Cooper and co-workers successfully synthesized a series of metal-free CMP photocatlysts by introducing photoactive molecules into CMP skeletons [22]. Rose Bengal (RB) has been extensively studied in the field of photocatalsis owing to its low cost and effective photocatalytic activity. CMPs could incorporate RB via the Sonogashira cross-coupling polymerization of terminal alkyne with iodine on RB. The RB-CMP displayed the typical micropore character, the large surface area up to ~800 m2/g, and the high photocatalytic activity for aza-Henry reaction under 60 W bulb light at room temperature. Afterwards, Cooper group synthesized a library of CMPs by the statistical copolymerization for varying optical gaps [23]. With increase of the incorporated pyrene chromophore, the fluorescence emission for these CMPs was tuned from blue to red color, and the corresponding optical gap was gradually shifted over the range of 1.94-2.95 eV. In the photocatalytic hydrogen-evolution experiments, it was found that the CMPs showed the higher activity under visible light than under UV light, while many organic photocatalysts perform actively only under UV light. Although the trace of Pd catalyst was remained in CMPs, the rate of hydrogen evolution strongly correlated with the optical gap of CMP. The average hydrogen evolution rate (17.4 ± 0.9 mmol h-1) given by the CMPs was significantly higher than that of other known photoactive polymers such as poly(azo- methine) networks and graphitic carbon nitride.
Vilela et al. designed a CMP network consisting of the electron- withdrawing benzothiadiazole as nodes and the weak electron- donating benzene as struts [24]. This combination facilitated the attenuation of exciton recombination and the increase of intersystem crossing to the triplet state for efficient singlet oxygen production. SiO2 nanoparticles served as templates to control the surface area and pore volume of CMPs. The photocatalysis results revealed that the CMPs were highly efficient and steadily reusable for singlet oxygen production. The conversions higher than 50% and selectivity above 80% could be achieved after a simple filtration. Furthermore, Vilela et al. modified the benzothiadiazole- based CMP by thiol-yne chemistry for increment of its hydrophi- licity [25]. The modified CMP was water-dispersible and could be used for the photocatalytic conversion of furoic acid to 5-hydroxy- 2(5H)-furanone with high conversions (~90%).
Zhang et al. proposed a structural design principle for CMPs' photocatalytic systems [26], which could enhance the catalytic activity by finely configuring the valence and conduction band levels (Fig. 7). Specifically, without changing the electron donor and acceptor moieties in the polymeric backbone, it appeared to simply vary the substitution positions of the electron acceptor on the centered phenyl units. It was reported that the benzoxadia- zole-based CMP with substitutions on the 1, 3, 5-positions of phenyl center had the better photocatalytic activity in the oxidative coupling of amines under a 23 W energy saving light bulb.

|
Download:
|
| Figure 7. Geometry design of benzooxadiazole-based CMPs for adjusting the valence and conduction bands [26]. | |
7. Phototherapy
Phototherapy is a comparatively noninvasive therapy, e.g. photothermal therapy (PTT) and photodynamic therapy (PDT), which involve systemic or topical administration and illumination of photosensitizers by low-intensity light. In contrast to the inorganic photosensitizers, use of conjugated polymers for phototherapy has gained increasing attention due to their biocompatibility and controllability in synthesis. As aforementioned, CMP has been a versatile platform for the wide applications. In our group, we proved the potential of nanoscae CMPs in PTT for killing cancer cells [27]. The synthesis was based on the uniform poly(methacrylic acid) (PMAA) microspheres, which were employed as templates for evolution of PPE-based CMP nanoshell by a template-mediated precipitation polymerization (Fig. 8a). Since PMAA could be decomposed during the reaction, the hollow cavity was concomitantly created, giving rise to a CMP microcapsule in one step. Modulation of the thickness and surface morphology of CMP shells was significant to improve the light absorption capability and photothermal conversion efficiency. It was found that the flower-like CMP microcapsules with a shell thickness of 100 nm showed the relatively high absorption in the near infrared region. Upon exposure to an 808-nm laser with a power density of 5 W/cm2, they were enabled to generate great heat in water, leading to a rising temperature of 24 ℃ in 6 min. The cell experiments demonstrated again that the photothermal conversion occurring on CMPs caused the thermal ablation of Hela cells with less than 10% viability (Fig. 8b-d). Compared with the linear conjugated polymer photosensitizers, CMPs not only display the enhanced heat radiation ability and the rapid response to near infrared light, but also possess the capability of drug loading for multimodal cancer treatment.

|
Download:
|
| Figure 8. (a) Preparation of different CMP nanostructures by using the PMAA microspheres as self-sacrincial templates. Fluorescence images ofHeLa cells treated by (b) laser (808 nm,5 W/cm2,5 min); (c) F-NCMP-100 (200 mg/mL); (d) laser (808 nm,5 W/cm2,5 min)+ F-NCMP-100 (200 mg/mL). Live and dead cells stained by calcein AM and propidium iodide dyes show the green and red colors,respectively [27]. | |
Although there has been no work yet on study of CMPs for PDT treatment of cancer cells, Han group has demonstrated the ability of metallophthalocyanine-based CMPs in generation of reactive oxide species [28]. In the system, metallophthalocyanine units were spatially isolated in the CMP network and their π-π interaction with each other was largely restricted. Additionally, due to the extended π-conjugation, the light harvesting capability was also enhanced, especially in the far-red window. By using1, 3- diphenylisobenzofuran as trap, it was demonstrated that the polymers were efficient for generation of singlet oxygen with irradiation of 700 nm light; the photoactivity was not only far higher than that of the corresponding monomers but nearly one order of magnitude higher than those of the reported organic porous materials. The excellent performance indicates the great potential of CMPs as attractive candidates for PDT agent.
8. ConclusionIn this review, we have described the recent application progress of photo-functional CMPs in many advanced fields including light harvesting/emission, chemosensing, photocatalysis, phototherapy, and optoelectronic devices. As compared with the non-porous analogues, CMP is a three-dimensional covalent network that is constructed by the large π-conjugation skeleton, in which the functional modules are spatially isolated and topologically connected to create large interfaces and nanopores. It is thus evident that CMPs exhibit the outstanding ability of exciton migration and carrier transport over the network backbone, and are enabled to promptly make response to the surrounding stimuli or specific chemicals confined in nanopores. Besides, the advantage in design and synthesis for CMPs provide opportunity to modulate their applicability and functionality at the molecular level. It could be anticipated that CMPs would continuously showcase their incredible potentials for tackling the environmental, energy and healthcare issues.
Acknowledgment The authors acknowledge the financial support of the NSFC (No. 21474015) and STCSM (No. 14ZR1402300).| [1] | X. Feng, X.S. Ding, D.L. Jiang. Covalent organic frameworks. Chem. Soc. Rev. 41 (2012) 6010–6022. DOI:10.1039/c2cs35157a |
| [2] | N.B. McKeown, P.M. Budd. Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 35 (2006) 675–683. DOI:10.1039/b600349d |
| [3] | M.P. Tsyurupa, V.A. Davankov. Porous structure of hypercrosslinked polystyrene: state-of-the-art mini-review. React. Funct. Polym. 66 (2006) 768–779. DOI:10.1016/j.reactfunctpolym.2005.11.004 |
| [4] | J.X. Jiang, F.B. Su, A. Trewin, et al. Conjugated microporous poly (aryleneethynylene) networks. Angew. Chem. Int. Ed. 46 (2007) 8574–8578. DOI:10.1002/anie.v46:45 |
| [5] | Y.H. Xu, S.B. Jin, H. Xu, A. Nagai, D.L. Jiang. Conjugated microporous polymers: design, synthesis and application. Chem. Soc. Rev. 42 (2013) 8012–8031. DOI:10.1039/c3cs60160a |
| [6] | K.Y. Wu, J. Guo. Controllable synthesis of multi-scale conjugated microporous polymer. Acta Chim. Sinica 73 (2015) 480–486. DOI:10.6023/A15020138 |
| [7] | J. Weber, A. Thomas. Toward stable interfaces in conjugated polymers: microporous poly (p-phenylene) and poly (phenyleneethynylene) based on a spirobifluorene building block. J. Am. Chem. Soc. 130 (2008) 6334–6335. DOI:10.1021/ja801691x |
| [8] | J.X. Jiang, A. Trewin, D.J. Adams, A.I. Cooper. Band gap engineering in fluorescent conjugated microporous polymers. Chem. Sci. 2 (2011) 1777–1781. DOI:10.1039/c1sc00329a |
| [9] | Y.H. Xu, L. Chen, Z.Q. Guo, A. Nagai, D.L. Jiang. Light-emitting conjugated polymers with microporous network architecture: interweaving scaffold promotes electronic conjugation, facilitates exciton migration, and improves luminescence. J. Am. Chem. Soc. 13 (2011) 17622–17625. |
| [10] | Y.H. Xu, A. Nagai, D.L. Jiang. Core-shell conjugated microporous polymers: a new strategy for exploring color-tunable and controllable light emissions. Chem. Commun. 49 (2013) 1591–1593. DOI:10.1039/C2CC38211C |
| [11] | X.M. Liu, Y.H. Xu, D.L. Jiang. Conjugated microporous polymers as molecular sensing devices: microporous architecture enables rapid response and enhances sensitivity in fluorescence-on and fluorescence-off sensing. J. Am. Chem. Soc. 134 (2012) 8738–8741. DOI:10.1021/ja303448r |
| [12] | C. Gu, N. Huang, J. Gao, et al. Controlled synthesis of conjugated microporous polymer films: versatile platforms for highly sensitive and label-free chemo-and biosensing. Angew. Chem. Int. Ed. 53 (2014) 4850–4855. DOI:10.1002/anie.201402141 |
| [13] | P. Zhang, J. Guo, C.C. Wang. Magnetic CMP microspheres: multifunctional poly (phenylene ethynylene) frameworks with covalently built-in Fe3O4 nanocrystals exhibiting pronounced sensitivity for acetaminophen microdetection. J. Mater. Chem. 22 (2012) 21426–21433. DOI:10.1039/c2jm34725c |
| [14] | K.Y. Wu, J. Guo, C.C. Wang. Dispersible and discrete metalloporphyrin-based CMP nanoparticles enabling colorimetric detection and quantitation of gaseous SO2. Chem. Commun. 50 (2014) 695–697. DOI:10.1039/C3CC47234E |
| [15] | J.M. Serin, D.W. Brousmiche, J.M.J. Fréchet. Cascade energy transfer in a conformationally mobile multichromophoric dendrimer. Chem. Commun. (2002) 2605–2607. |
| [16] | L. Chen, Y. Honsho, S. Seki, D.L. Jiang. Light-harvesting conjugated microporous polymers: rapid and highly efficient flow of light energy with a porous polyphenylene framework as antenna. J. Am. Chem. Soc. 132 (2010) 6742–6748. DOI:10.1021/ja100327h |
| [17] | C. Gu, N. Huang, F. Xu, J. Gao, D.L. Jiang. Cascade exciton-pumping engines with manipulated speed and efficiency inlight-harvesting porous p-network films. Sci. Rep. 5 (2015) 8867. DOI:10.1038/srep08867 |
| [18] | P. Zhang, K.Y. Wu, J. Guo, C.C. Wang. From hyperbranched polymer to nanoscale CMP (NCMP): Improved microscopic porosity, enhanced light harvesting, and enabled solution processing into white-emitting Dye@NCMP films. ACS Macro Lett. 3 (2014) 1139–1144. DOI:10.1021/mz5005508 |
| [19] | C. Gu, Y.C. Chen, Z.B. Zhang, et al. Electrochemical route to fabricate film-like conjugated microporous polymers and application for organic electronics. Adv. Mater. 25 (2013) 3443–3448. DOI:10.1002/adma.v25.25 |
| [20] | C. Gu, N. Huang, Y.C. Chen, et al. π-Conjugated microporous polymer films: designed synthesis, conducting properties, and photoenergy conversions. Angew. Chem. Int. Ed. 54 (2015) 13594–13598. DOI:10.1002/anie.201506570 |
| [21] | C. Gu, N. Huang, Y.C. Chen, et al. Porous organic polymer films with tunable work functions and selective hole and electron flows for energy conversions. Angew. Chem. Int. Ed. 128 (2016) 3101–3105. DOI:10.1002/ange.201510723 |
| [22] | J.X. Jiang, Y.Y. Li, X.F. Wu, et al. Conjugated microporous polymers with rose bengal dye for highly efficient heterogeneous organo-photocatalysis. Macromolecules 46 (2013) 8779–8783. DOI:10.1021/ma402104h |
| [23] | R.S. Sprick, J.X. Jiang, B. Bonillo, et al. Tunable organic photocatalysts for visiblelight-driven hydrogen evolution. J. Am. Chem. Soc. 137 (2015) 3265–3270. DOI:10.1021/ja511552k |
| [24] | K. Zhang, D. Kopetzki, P.H. Seeberger, M. Antonietti, F. Vilela. Surface area control and photocatalytic activity of conjugated microporous poly(benzothiadiazole) networks. Angew. Chem. Int. Ed. 52 (2013) 1432–1436. DOI:10.1002/anie.201207163 |
| [25] | H. Urakami, K. Zhang, F. Vilela. Modification of conjugated microporous polybenzothiadiazole for photosensitized singlet oxygen generation in water. Chem. Commun. 49 (2013) 2353–2355. DOI:10.1039/c3cc38956a |
| [26] | Z.J. Wang, S. Ghasimi, K. Landfester, K.A.I. Zhang. Molecular structural design of conjugated microporous poly(benzooxadiazole) networks for enhanced photocatalytic activity with visible light. Adv. Mater. 27 (2015) 6265–6270. DOI:10.1002/adma.201502735 |
| [27] | J. Tan, J.X. Wan, J. Guo, C.C. Wang. Self-sacrificial template-induced modulation of conjugated microporous polymer microcapsules and shape-dependent enhanced photothermal efficiency for ablation of cancer cells. Chem. Commun. 51 (2015) 17394–17397. DOI:10.1039/C5CC05478H |
| [28] | X.S. Ding, B.H. Han. Metallophthalocyanine-based conjugated microporous polymers as highly efficient photosensitizers for singlet oxygen generation. Angew. Chem. Int. Ed. 54 (2015) 6536–6539. DOI:10.1002/anie.201501732 |
 2016, Vol. 27
2016, Vol. 27 


