Covalent organic frameworks (COFs) are a class of porous crystalline materials which consist of organic building blocks orderly linked by strong covalent bonds [1], and it can also be defined as a type of two-dimensional polymer. According to the principles of reticular chemistry, the porosity and composition of COFs can be well controlled. That they are composed of lightweight elements bestows the character of low mass density on COFs, and covalent bonds linkages endow COFs with thermal stabilities. Combining the advantages of permanent porosity and high surface area makes COFs a perfect candidate material for gas storage [2], gas separation [3], catalysis [4], and proton conductivity [5]. Moreover, another significant feature of COFs is that their functionality can be tuned by carefully choosing the type of the building blocks, resulting in potential applications in various fields. Polymers with π-conjugated functional groups usually possess fluorescent and photoconductive property [6]. Similarly, if we use π-conjugated molecules as the monomers, COFs with photoconductive properties can be obtained as well [7]. Fig. 1 summarizes the π-functional molecules which can be used for synthesizing the semiconducting COFs. In this short review, we summarize the recent advance in this field.
|
Download:
|
| Figure 1. π-functional molecules used for synthesizing semiconducting COFs. | |
2. Fundamentals and motivation
COFs can be developed into two-dimensional (2D) or threedimensional (3D) frameworks based on the structure of building blocks. In 2005, the first 2D COFs were designed and synthesized through the condensation reaction of bor- onate ester [1]. To improve the crystallization, the organic reaction for synthesizing COFs need have a good reversibility [8], and till now the reactions include the formation of B-O (boronate, boroxine, and borosilicate) [9], C=N (imine, hydrazine, and squaraine) [10], C-N (triazine and imide) [11], B-N (borazine) [12], and N=N (azodioxide) [13] bond linkages (Fig. 2).

|
Download:
|
| Figure 2. Linkages and organic reactions used for synthesizing COFs. | |
In 2D COFs, organic building blocks are orderly linked to form 2D sheets with regular pores which then stack together by π-π interaction of the building blocks in a face-to-face way. So, there are periodically aligned columns developed by building blocks in 2D COFs. Such a well-organized structure will benefit 2D COFs applied as organic semiconductors when selecting π-functional molecules as building blocks, because the charge carriers excited from the building blocks will transport along the discrete and periodic columns [14]. These unidirectional pathways for charge carrier transport definitely improve the charge carrier mobility of 2D COFs as semiconductor. That is a great advantage compared with traditional organic semiconductors or polymer semiconductors which are amorphous.
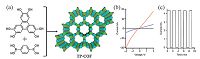
3. P-type semiconducting COFsUtilizing π-functional organic molecules especially with electron-rich feature to construct COFs which will form an extended π-conjugated system, renders the COFs p-type semiconducting behavior. In 2008, Jiang et al. reported the first example of semiconducting TP-COFs, with a pore size of 3.14 nm, by condensation reaction of 2, 3, 6, 7, 10, 11-hexahydroxytriphenylene (HHTP) and pyrene-2, 7-diboronic acid (PDBA) (Fig. 3a) [15]. The specific surface area and pore volume values of TP-COFs were 868 m2g-1 and 0.7907 cm3 g-1, respectively. The electrical conductivity of TP-COFs was measured by a two-probe method. The homogenous acetone dispersion of TP-COF was casted onto the Pt electrodes with the width of 10 mm. The I-V profile of TP-COF was linear in the air at 25 ℃, while the gap itself was silent which meat TP-COF had a semiconductor character. After TP-COF was doped with iodine, the electric current increased to 4.3 nA at 2 V bias voltage, suggesting that TP-COF was a p-type organic semiconductor (Fig. 3b). It showed a good stability, and the electric current did not deteriorate after switched on-off many times (Fig. 3c).
|
Download:
|
| Figure 3. (a) Schematic representation of TP-COF’s synthesis and structure (structure is based on quantum calculation and crystal lattice parameters; B purple, O red, triphenylene green, pyrene blue, H atoms are omitted for clarity). (b) I–V profile of a 10 μm width Pt gap (black curve: without TP-COF; blue curve: with TP-COF; red curve: with iodine-doped TP-COF). (c) Electric current when 2 V bias voltage is turned on or off. (Adapted with permission from Ref. [15]. Copyright 2008 Wiley-VCH). | |
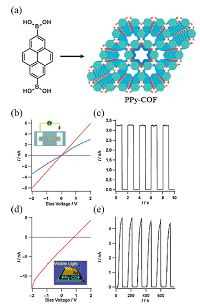
Jiang et al. pioneered the research of photoconductive COFs by embedding pyrene to obtain PPy-COF via the self-condensation of pyrene-2, 7-diboronic acid (PDBA) (Fig. 4a) [16]. PPy-COF has a pore diameter of 1.73 nm and a surface area of 923 m2 g-1, and shows an electrical conductivity similar with TP-COF (Fig. 4b and c). To investigate its photoconductivity behavior, PPy-COF thin film was sandwiched between Al and Au electrodes. When visible light (> 400 nm) irradiated from the Au side, a remarkable photocurrent is generated (Fig. 4d), and it can be switched repetitively by many times without any deterioration at an on-off ratio over 8.0 × 104 (Fig. 4e).
|
Download:
|
| Figure 4. (a) Schematic representation of PPy-COF’s synthesis and structure (The structure is based on quantum-chemical calculations and crystal-lattice parameters; B white, O red, pyrene blue; H atoms are omitted for clarity). b) I–V profile of PPy-COF between two Pt electrodes 10 mm apart black curve: without PPy-COF; blue curve: with PPy-COF; red curve: with iodine-doped PPy-COF. c) Electric current when the 2 V bias voltage was turned on or off. d) I–V profile of PPy-COF between sandwich-type Al/Au electrodes (black curve: without light irradiation; red curve: upon light irradiation. e) Photocurrent when the light was turned on or off. (Adapted with permission from Ref. [16]. Copyright 2009 Wiley-VCH). | |
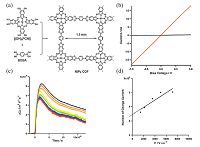
Choosing large and planar conjugated monomers as the building block will definitely increase the electronic conductivity of COFs. For instance, phthalocyanines and porphyrins are extensively adopted to synthesize semiconducting COFs [17]. In 2011, Jiang et al. and Yaghi et al. reported the phthalocyanine- based and porphyrin-based COFs by solvothermal method, respectively [14, 18]. (2, 3, 9, 10, 16, 17, 23, 24-octahydroxyphthalo- cyaninato)nickel(II), [(OH)8PcNi], and 1, 4-benzenediboronic acid (BDBA) reacted in dimethylacetamide (DMAc)/o-dichlorobenzene via boronate esterification reaction, forming crystalline NiPc COF in high yield (Fig. 5a) [18]. NiPc COF exhibits a linear I-V curve when it is sandwiched between Au and Al electrodes (Fig. 5b), suggesting its perfect conductive property. Its intrinsic charge-carrier mobility was measured by laser flash-photolysis time-resolved microwave conductivity measurements (FP-TRMC) (Fig. 5c and d). NiPc COF's carrier species was demonstrated to originate from holes with a mobility as high as 1.3 cm2 V-1 s-1. They also found that, due to the central metal's influence on the π-electron density of metallophthalocyanine, the metal species could significantly influence the carrier mobilities of the phthalocyanine-based COFs (CuPc-COF < ZnPc-COF < CoPc-COF) [19]. Moreover, Yaghi et al. synthesized porphyrin-based COFs (COF-366 and COF-66) by Schiff Base reaction and boronate esterification reaction (Fig. 6a and b) [14]. Compared with COF-66 (hole mobility 3.0 cm2 V-1 s-1), COF-366 has a much higher hole mobility (8.1 cm2V-1 s-1), which is also the highest in semiconducting COFs till now (Fig. 6c and d). It should be attributed to the linkage of imine in COF-66, which improves the conjugacy of the frameworks.
|
Download:
|
| Figure 5. (a) The synthesis of the nickel phthalocyanine covalent organic framework (NiPc COF) by a boronate esterification reaction. (b) I-V curves of NiPc COF (red curve) and [(MeO)8PcNi] (black curve) sandwiched between Al/Au electrodes. (c)|Transient conductivity profiles upon irradiation with a 355|nm pulsed laser at different photon densities: 4.5 × 1014 (red curve) to 1.5 × 1016 photon cm -2 (black curve). (d) Number of charge carriers measured by the time-of-flight transient current integration at different bias voltages, irradiated with a 355 nm pulsed laser at 6.5 × 1014 photon cm-2. (Adapted with permission from Ref. [18]. Copyright 2011 Wiley-VCH). | |

|
Download:
|
| Figure 6. The synthesis of (a) COF-366 and (b) COF-66. (c) FP TRMC profile of COF-366 (red) and COF-66 (blue) at 25 ℃ upon irradiation with a 355 nm pulse laser at a power of 1.4 × 1016 and 2.1 × 1016 photons cm -2,respectively. (d) Accumulated number of photo-induced charge carriers upon 355 nm pulse exposure to COF-366 (red)/COF-66 (blue) sandwiched by ITO and Al electrodes. Excitation was carried out at the photon density of 9. (Adapted with permission from Ref. [14]. Copyright 2011 American Chemical Society). | |
Tetrathiafulvalene (TTF) is a typical organic semiconductor molecule with a strong electron donor behavior. COFs with TTF building block were synthesized by reacting 2, 3, 6, 7-tetra(4- formylphenyl) tetrathiafulvalene with 1, 4-phenyldiamine or 1, 3, 6, 8-tetra(4-aminophenyl)pyrene (Fig. 7a) in 2014 [20]. TTF- Ph-COF and TTF-Py-COF are not only thermally stable (no obvious weight loss until 450 ℃), but also have a good chemical stability in organic solvents. TTF-Ph-COF is a mesoporous material with pore size of 2.2 nm and surface area as high as 1014 m2 g-1, while TTF- Py-COF is a microporous material with pore size of 1.3 nm and surface area of 817 m2 g-1. TTF itself is a hole-transporting material, so TTF-based COFs are p-type semiconductors. The carrier mobilities of TTF-Ph-COF and TTF-Py-COF are 0.2 and 0.08 cm2 V-1 s-1, respectively. Moreover, when doping TTF-based COFs with electron acceptors such as I2 or tetracyanoquinodi- methane (TCNQ), their conductivity can be improved significantly (Fig. 7b-e) [21].

|
Download:
|
| Figure 7. (a) Schematic representation of the synthesis of mesoporous TTF-Ph-COF and microporous TTF-Py-COF. I-V curves of (b) TTF-Ph-COF and (d)|TTF-Py-COF (black curves: pristine COFs, red and blue curves: COFs upon iodine oxidation). Time-dependent current of (c) TTF-Ph-COF and (e) TTF-Py-COF upon iodine oxidation. (Adapted with permission from Ref. [21]. Copyright 2014 Wiley-VCH). | |
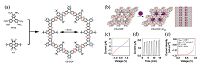
Due to blocking by the linkages, typical COFs lack intra-sheet π- conjugation. The fully intra-sheet π-conjugated COFs will definitely increase both the chemical stability and the electrical conductivity. In 2013, Jiang et al. used triphenylene hexamine (TPHA) and tert-butylpyrene tetraone (PT) as the monomers to synthesize the crystalline phenazine-linked CS-COF through topological ring fusion reaction (Fig. 8a) [22]. Owing to the π-electron delocalization, CS-COF performs high-rate hole-conducting with mobility up to 4.2 cm2 V-1 s-1. Furthermore, they loaded fullerene into the pores of CS-COF forming a donor-acceptor system (CS-COF⊃C60) (Fig. 8b). The sandwiched devices with CS- COF⊃C60 as the photoconductive layer display a rapid response to light irradiation with an on-off ratio up to 5.9 × 107 (Fig. 8c and d). When using CS-COF⊃C60 in solar cells, the power conversion efficiency is about 0.9% (Fig. 8e). Bein et al. reported a thienothiophene-based covalent organic (TT-COF) [23]. After loading TT-COF with electron acceptor [6,6]-phenyl-C61-butyric acid methyl ester (PCBM), it can be used in solar cells with a power conversion efficiency only about 0.053%, much lower than the fully intra-sheet π-conjugated COFs.
|
Download:
|
| Figure 8. (a) Schematic representation of the synthesis of CS-COF. The dotted blue lines on the periphery imply the extension of periodic structures. (b) Schematic representation of synthesis of CS-CS-COF⊃C60 by sublimed crystallization of fullerenes in the open one-dimensional channels (white: carbon; red: nitrogen; purple: fullerene). A side view of CS- CS-COF⊃C60 is also shown. (c) I-V curve of a 50-nm-thick CS-CS-COF⊃C60/PMMA film sandwiched between Al and Au electrodes at bias voltages ranging from -1.5Vto1.5Vin air at 25℃. (d) Photocurrent switching at a bias voltage of1.5V in air at 25℃,with repetitive light on-off actions on the 50-nm-thick CS-COF⊃C60/PMMA film. (e) J-V curve of the photovoltaic cell under irradiation with air mass 1.5 conditions (VOC = 0.98 V,JSC = 1.7 mA cm-2,FF = 0.54) . (Adapted with permission from Ref. [22]. Copyright 2013 Nature Publishing Group). | |
4. Ambipolar semiconducting COFs
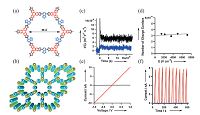
Ambipolar semiconducting COFs usually have both electron donor (D) and electron acceptor (A) building blocks, which can be obtained by post-synthesis functionalizing or directly using D and A monomers in the synthesis [24]. Utilizing two different kinds of building blocks, D and A molecules, to construct COFs will form a segregated and bi-continuous D-A structure, which produces periodic and unidirectional D-on-D and A-on-A columnar arrays. Compared with the amorphous D-A polymers in which D and A units stack disorderly, D-A COFs have a better control on the charge dynamics. The first example of D-A COFs was reported in 2012, in which 2, 3, 6, 7, 10, 11-hexahydroxytriphenylene (HHTP) was used as the electron donor and 2, 1, 3-benzothiadiazole-4, 7- diboronic acid (BTDADA) was used as the electron donor (Fig. 9a and b) [25]. The D column is the pathway for hole transport, while the A column is for electron transport. It hole and electron mobilities were measured by FP-TRMC method, which were 0.01 and 0.04 cm2 V-1 s-1, respectively (Fig. 9c and d). A photocurrent with linear I-V curve was observed when the D-A COF was irradiated by visible light (Fig. 9e), whereas the mixture of D and A molecule with a molar ratio of 3:2 did not response to light irradiation. Furthermore, this D-A COF has a quick response to light irradiation, and the photocurrent can be switched on and off many times without deterioration as shown in Fig. 9f.
|
Download:
|
| Figure 9. Schematic representation of 2D D-A COF with self-sorted and periodic electron donor-accepter ordering and bicontinuous conducting channels. (a) right: stru one hexagon; (b) left: a 3 × 3 grid. (c) FP-TRMC profiles of the 2D D-A COF in Ar (black) and SF6 atmospheres (blue). (d) Accumulated number of photo-induced charge in the 2D D-A COF upon 355 nm pulse exposure. (e) I-V curves of the 2D D-A COF in the dark (black) and upon irradiation (red) with visible light from a Xenon light so Photocurrents of the 2D D-A COF (red curve),COF-5 (black),and a simple mixture of D and A at a molar ratio of 3:2 (blue) upon repeated switching of the light on (Adapted with permission from Ref. [25]. Copyright 2012 Wiley-VCH). | |
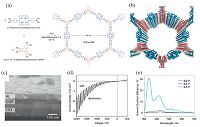
Bein et al. developed a triphenylene-porphyrin (TP-Por) COF with highly defined interdigitated donor-acceptor heterojunctions, and grew an oriented TP-Por COF thin film on ITO-covered glass (Fig. 10a and b) [26]. Using the thin film, they fabricated a photovoltaic device in which the COF itself provided the photoactive junction and investigated the charge carrier generation and extraction (Fig. 10c). The D and A units in the TP-Por COF film formed one-dimensional stacked columns that extended along the substrate. The short-circuit current was about 30 times higher than that of a reference device based on a randomly intermixed blend of the two building blocks (Fig. 10d). Moreover, the external quantum efficiency of this COF-based device could be boosted to more than 30% at 350 nm under reverse bias, which is highly enough to replace small molecule/fullerene-containing devices (Fig. 10e).
|
Download:
|
| Figure 10. (a) Co-condensation of bis(boronophenyl)porphyrin and HHTP leading to the formation of the layered TP-Por COF. The COF features hexagonal pores with a large diameter of 4.6 nm. (b) Illustration of the TP-Por COF highlighting the alternating columns of triphenylene (red) and porphyrin (blue) subunits. (c) Cross-sectional scanning electron micrograph of a TP-Por COF-based photovoltaic device showing the COF layer between the ITO and Al electrodes. The MoOx and ZnO contact layers are too thin to be visible in the micrograph. (d) Current density-voltage curves for the photovoltaic device under chopped white-light illumination. (e) Bias-dependent EQE spectra illustrating the greatly enhanced charge collection efficiency under reverse-bias conditions. (Adapted with permission from Ref. [26]. Copyright 2013 American Chemical Society). | |
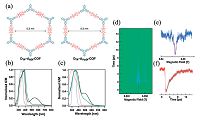
In 2013, two kinds of D-A COFs with the largest pore size among known COFs, as large as 5.3 nm, were reported [27]. Triphenylene and diimide were selected as vertices and edge units respectively to construct hexagonal triphenylene-diimide COFs (Fig. 11a, DTP- ANDI-COF and DTP-APyrDI-COF). The charge transfer was detected by steady-state electronic absorption spectra. DTP-APyrDI-COF exhibited a clear charge-transfer (Fig. 11c), while no clear charge- transfer band was observed for Dtp-Andi-COF (Fig. 11b). It is because that the D and A columns in DTP-ANDI-COF are indepe at the ground state leading to a neutral system ready for separation. The lifetime of charge-separated was also measu time resolved electron spin resonance spectroscopy (TR-E track the formation of radical spins and their dynamics. irradiation by laser (355 nm, 2 mJ), the TR-ESR signal of DTP COF increases rapidly to 2.5 μs, as the result of charge sepa (Fig. 11d-f). However, DTP-APyrDI-COF did not have any sign the TR-ESR measurements of which reason may be the ac structure. That means pairing donor and acceptor is a key fa design COFs for solar energy conversion.
|
Download:
|
| Figure 11. (a) Schematic representation of the structures of DTP-ANDI-COF and DTP-APyrDI-COF. Dotted lines at the periphery indicate extended structure. Electronic absorption spectra of (b) DTP-ANDI (green) and (c) DTP-APyrDI-COF (green), simple mixtures of their monomers (black), TP(OMe)6 (dotted blue), and NDI boronate ester (dotted red), PyrDI boronate ester (dotted red). (d) Contour plots of the TR-ESR spectrum of the solid-state DTP-ANDI at 80 K. The transverse and longitudinal axes denote the time and magnetic field, respectively. The normal axis is the TR-ESR intensity. The negative and positive signs of the signal intensity indicate the absorption and emission of the microwaves, respectively. (e) Time-slice of the TR-ESR spectrum at a time of 2.5 μs. The red line is the line calculated based on the Lorentz function. (f) Time profile of the TR- ESR signal at a magnetic field of 0.3464 T. The sky-blue line is the curve calculated based on the exponential function Φ =α exp[-t/τCS]. (Adapted with permission from Ref. [27]. Copyright The Royal Society of Chemistry 2013) . | |
To further investigate the effect of different electron don acceptor pairs on COFs' charge transfer and separation, et al. employed different electron-donating metallophtha nines and electron-accepting diimide derivatives to constru COFs (Fig. 12), and showed that the metal species in phtha nines and the class of acceptor could influence the photoch dynamics of the D-A COFs [28].

|
Download:
|
| Figure 12. Schematic representation of the donor-acceptor COFs (DMPC-ADI-COFs) with covalently linked phthalocyaninediimide (MPc-DI) structures. (Adapted with permission from Ref. [28]. Copyright 2013 American Chemical Society). | |
5. n-type semiconducting COFs
p-type and ambipolar semiconducting COFs have been si intensively; however, only a few n-type semiconducting were reported. In 2011, Jiang et al. reported an n-ch conducting COF with high electron mobility and prom photoconductivity [29]. Their strategy was to employ ele withdrawing building block, benzothiadiazole (BTDA), at the of a two-component tetragonal nickel(II) phthalocyanine CO NiPc-BTDA COF) (Fig. 13a), which gave rise to a drastic cha the carrier-transport mode from hole-transporting to ele conducting. The 2D-NiPc-BTDA COF was synthesized by co sation of (2, 3, 9, 10, 16, 17, 23, 24-octahydroxyphthalocyani nickel(II) ([OH]8PcNi) and BTDADA in a mixture dichlorobenzene and dimethylacetamide under solvoth condition in 85% isolated yield. 2D-NiPc-BTDA COF has tetr pores with a pore size of 2.2 nm and its surface area was evaluated to be 877 m2g-1. Its intrinsic carrier mobility was measured by FP-TRMC method under different atmospheres, which demonstrated an n-type semiconducting behavior with electron mobility as high as 0.6 cm2 V-1 s-1 (Fig. 13b and c). When irradiating the 2D-NiPc-BTDA COF by light with different wavelengths, obvious photoconductive behavior was detected, and it could be switched on-off by many times without any deterioration (Fig. 13d and e).

|
Download:
|
| Figure 13. (a) Schematic representation of the synthesis of 2D-NiPc-BTDA COF with metallophthalocyanine at the vertices and BTDA at the edges of the tetragonal framework. (b) Transient conductivities irradiated with a 355-nm pulsed laser at the photon density of 1.6 × 1015 under Ar (red curve), SF6 (blue curve), and after annealing under SF6 for 1 h (orange curve) and 24 h (green curve) or under O2 for 24 h (black curve). (c) Number of charge carriers measured by the time-off light transient current integration at different bias voltages, irradiated with a 355-nm pulsed laser of 5.0 × 1014 photons cm-2. (d) On-off switching of photocurrent of 2D-NiPc-BTDA COFs at the bias voltage of 1.0 V, by switching the light on and off. (e) Wavelength-dependent on-off switching of photocurrent at the bias voltage of 1.0 V. (Adapted with permission from Ref. [29]. Copyright 2011 American Chemical Society). | |
Another example of n-type semiconducting COFs is Cu porphyrin-based COF (CuP-COF), which has minimum electron mobilities up to 0.19 cm2 V-1 s-1 [30]. Interestingly, removing or changing the central metal of the building block of porphyrin would switch conducting nature of the porphyrin-based COF (MP- COF, M = H2, Zn, and Cu) (Fig. 14a). The stacking macrocycles of H2P-COF without central metals formed a path for the hole transport. When inserting a metal species into the porphyrin macrocycles, it formed another path, metal-on-metal channel, for the electron transport (Fig. 14c). The insertion of a central metal to the porphyrin macrocycle lowered the electron density of the macrocycles. According to the central metal's ability of exhibiting ligand-to-metal charge transfer, copper metal reduced much more electron density of the porphyrin macrocycles. Thus, CuP-COF favored electron transport through the metal-on-metal channel, whereas ZnP-COF allowed balanced transport through both channels (Fig. 14b). However, ZnP-COF exhibited better photoconductivity than CuP-COF with an on-off ratio of 5 × 104(Fig. 14d and e).

|
Download:
|
| Figure 14. (a) Schematic representation of MP-COFs (M = H2, Zn, and Cu). (b) Schematic graphs of a 2 × 2 grid of MP-COFs with achiral AA stacking 2D sheets (C: light blue; N: deep blue; H: white; O: red; B: pink; Zn: green; Cu: violet). (c) Graphical representation of metal-on-metal and macrocycle-on-macrocycle channels for respective electron and hole transport in stacked porphyrin column of 2D porphyrin COFs. (d) Photocurrents for 2D porphyrin COFs upon repeated switching of the light on and off. (e) Wavelength-dependent on-off switching of photocurrent of ZnP-COF at a bias voltage of 1.0 V. (Adapted with permission from Ref. [30]. Copyright 2012 Wiley-VCH). | |
6. Conclusion
In summary, this mini review summarized different types of semiconducting COFs and the strategies to choose π-functional building blocks. COF materials themselves possess the advantages of low density, large surface areas and permanent porosity. The most important one is that their properties and functionalities can be tuned by the building blocks and covalent linkages. Using building blocks with rich conjugated π-electron tend to obtain p- type semiconducting COFs, whereas integrating electron-withdrawing building blocks can switch them to ambipolar or n-type semiconductors. Because of their ordered structure, the charge mobilities are normally higher than amorphous conducting polymers and organic molecules, however some challenges still remain, including: (1) the study on semiconducting COFs is still confined to their semiconducting properties. Therefore, applying them on photoelectric devices is the primary challenge to overcome in future; (2) n-type semiconductor is an essential part in photovoltaic devices [31], but only two examples of n-type semiconducting COFs have been reported. Thus, more effort toward new n-type semiconducting COFs is required; (3) COFs materials are insoluble in various organic solvents, giving rise to a processing problem when fabricating the optoelectronic devices. To develop a reliable method to prepare COF thin films is of great importance for its practical application [32]; (4) in terms of the synthetic methods, crystalline COFs are usually synthesized by solvothermal method, which is hard to be scaled up and needs a long reaction time. To develop methods under mild reaction conditions for large scale production of crystalline COFs is still a big challenge [33]. We believe, as a novel type of 2D conducting polymers, the semiconducting COFs have great potential to be a new generation of organic semiconductors, and would have a promising future in the application of high performance optoelectronic devices.
Acknowledgment We are grateful to the support from National Program for Thousand Young Talents of China, the National Natural Science Foundation of China (No. 21544001), and Fudan University.| [1] | A.P. Côté, A.I. Benin, N.W. Ockwig, et al. Porous, crystalline, covalent organic frameworks. Science 310 (2005) 1166–1170. DOI:10.1126/science.1120411 |
| [2] | (a) H. Furukawa, O.M. Yaghi, Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications, J. Am. Chem. Soc. 131(2009) 8875-8883; (b) C.J. Doonan, D.J. Tranchemontagne, T.G. Glover, J.R. Hunt, O.M. Yaghi, Exceptional ammonia uptake by a covalent organic framework, Nat. Chem. 2(2010) 235-238. |
| [3] | (a) N. Huang, X. Chen, R. Krishna, D.L. Jiang, Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization, Angew. Chem. Int. Ed. 54(2015) 2986-2990; (b) M.M. Tong, Q.Y. Yang, Q.T. Ma, D.H. Liu, C.L. Zhong, Few-layered ultrathin covalent organic framework membranes for gas separation: a computational study, J. Mater. Chem. A 4(2016) 124-131. |
| [4] | (a) S.Y. Ding, J. Gao, Q. Wang, et al., Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction, J. Am. Chem. Soc. 133(2011) 19816-19822; (b) S. Lin, C.S. Diercks, Y.B. Zhang, et al., Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water, Science 349(2015) 1208-1213; (c) Y. Wu, H. Xu, X. Chen, J. Gao, D.L. Jiang, A (-electronic covalent organic framework catalyst: (-walls as catalytic beds for Diels-Alder reactions under ambient conditions, Chem. Commun. 51(2015) 10096-10098. |
| [5] | (a) X. Chen, M. Addicoat, S. Irle, A. Nagai, D.L. Jiang, Control of crystallinity and porosity of covalent organic frameworks by managing interlayer interactions based on self-complementary π-electronic force, J. Am. Chem. Soc. 135(2013) 546-549; (b) S. Chandra, T. Kundu, K. Dey, et al., Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks, Chem. Mater. 28(2016) 1489-1494; (c) H. Xu, S.S. Tao, D.L. Jiang, Proton conduction in crystalline and porous covalent organic frameworks, Nat. Mater. 15(2016) 722-726. |
| [6] | (a) G.Q. Lin, H.M. Ding, D.Q. Yuan, B.S. Wang, C. Wang, A pyrene-based, fluorescent three-dimensional covalent organic framework, J. Am. Chem. Soc. 138(2016) 3302-3305; (b) H. Chen, X. Xu, H. Gang, et al., Novel fluorene-carzazole-based conjugated copolymers containing pyrazoline and benzothiazole segments for blue lightemitting materials, Chin. Chem. Lett. 18(2007) 1496-1500;(c) Z. Wang, W. Zhang, F. Tao, et al., Synthesis and properties of two novel copolymers based on squaraine and fluorene units for solar cell materials, Chin. Chem. Lett. 22(2011) 1001-1004; (d) X.R. Cai, H. Chen, T. Zhang, et al., Synthesis and characterization of a novel main chain oxadiazole-based copolymer for n-type solar cell material, Chin. Chem. Lett. 18(2007) 1342-1346. |
| [7] | M.Dogru, T. Bein. On the road towards electroactive covalent organic frameworks. Chem. Commun. 50 (2014) 5531–5546. DOI:10.1039/C3CC46767H |
| [8] | P.J. Waller, F. Gándara, O.M. Yaghi. Chemistry of covalent organic frameworks. Acc. Chem. Res. 48 (2015) 3053–3063. DOI:10.1021/acs.accounts.5b00369 |
| [9] | (a) H.M. El-Kaderi, J.R. Hunt, J.L. Mendoza-Cortés, et al., Designed synthesis of 3D covalent organic frameworks, Science 316(2007) 268-272; (b) J.R. Hunt, C.J. Doonan, J.D. LeVangie, A.P. Côté, O.M. Yaghi, Reticular synthesis of covalent organic borosilicate frameworks, J. Am. Chem. Soc. 130(2008) 11872-11873. |
| [10] | (a) F.J. Uribe-Romo, J.R. Hunt, H. Furukawa, et al., A crystalline imine-linked 3-D porous covalent organic framework, J. Am. Chem. Soc. 131(2009) 4570-4571; (b) F.J. Uribe-Romo, C.J. Doonan, H. Furukawa, K. Oisaki, O.M. Yaghi, Crystalline covalent organic frameworks with hydrazone linkages, J. Am. Chem. Soc. 133(2011) 11478-11481; (c) A. Nagai, X. Chen, X. Feng, et al., A squaraine-linked mesoporous covalent organic framework, Angew. Chem. Int. Ed. 52(2013) 3770-3774. |
| [11] | (a) P. Kuhn, M. Antonietti, A. Thomas, Porous, covalent triazine-based frameworks prepared by ionothermal synthesis, Angew, Chem. Int. Ed. 47(2008) 3450-3453; (b) Q.R. Fang, Z.B. Zhuang, S. Gu, et al., Designed synthesis of large-pore crystalline polyimide covalent organic frameworks, Nat. Commun. 5(2014) 4503. |
| [12] | K.T. Jackson, T.E. Reich, H.M. El-Kaderi. Targeted synthesis of a porous borazinelinked covalent organic framework. Chem. Commun. 48 (2012) 8823–8825. DOI:10.1039/c2cc33583b |
| [13] | D. Beaudoin, T. Maris, J.D. Wuest. Constructing monocrystalline covalent organic networks by polymerization. Nat. Chem. 5 (2013) 830–834. DOI:10.1038/nchem.1730 |
| [14] | S. Wan, F. Gándara, A. Asano, et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23 (2011) 4094–4097. DOI:10.1021/cm201140r |
| [15] | S. Wan, J. Guo, J. Kim, H. Ihee, D.L. Jiang, A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 47 (2008) 8826–8830. DOI:10.1002/anie.v47:46 |
| [16] | S. Wan, J. Guo, J. Kim, H. Ihee, D.L. Jiang. A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. 48 (2009) 5439–5442. DOI:10.1002/anie.v48:30 |
| [17] | (a) H.P. Liao, H.M. Wang, H.M. Ding, et al., A 2D porous porphyrin-based covalent organic framework for sulfur storage in lithium-sulfur batteries, J. Mater. Chem. A 4(2016) 7416-7421; (b) H.M. Wang, H.M. Ding, X.S. Meng, C. Wang, Two-dimensional porphyrin- and phthalocyanine-based covalent organic frameworks, Chin. Chem. Lett. 27(2016) 1376-1382. |
| [18] | X.S. Ding, J. Guo, X. Feng, et al. Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. 50 (2011) 1289–1293. DOI:10.1002/anie.v50.6 |
| [19] | X.S. Ding, X. Feng, A. Saeki, et al. Conducting metallophthalocyanine 2D covalent organic frameworks: the role of central metals in controlling p-electronic functions. Chem. Commun. 48 (2012) 8952–8954. DOI:10.1039/c2cc33929c |
| [20] | S.B. Jin, T. Sakurai, T. Kowalczyk, et al. Two-dimensional tetrathiafulvalene covalent organic frameworks: towards latticed conductive organic salts. Chem. Eur. J. 20 (2014) 14608–14613. DOI:10.1002/chem.v20.45 |
| [21] | (a) H.M. Ding, Y.H. Li, H. Hu, et al., A tetrathiafulvalene-based electroactive covalent organic framework, Chem. Eur. J. 20(2014) 14614-14618; (b) S.L. Cai, Y.B. Zhang, A.B. Pun, et al., Tunable electrical conductivity in oriented thin films of tetrathiafulvalene-based covalent organic framework, Chem. Sci. 5(2014) 4693-4700. |
| [22] | J. Guo, Y.H. Xu, S.B. Jin, et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4 (2013) 2736. |
| [23] | M. Dogru, M. Handloser, F. Auras, et al. A photoconductive thienothiophenebased covalent organic framework showing charge transfer towards included fullerene. Angew. Chem. Int. Ed. 52 (2013) 2920–2924. DOI:10.1002/anie.201208514 |
| [24] | L. Chen, K. Furukawa, J. Gao, et al. Photoelectric covalent organic frameworks: converting open lattices into ordered donor-acceptor heterojunctions. J. Am. Chem. Soc. 136 (2014) 9806–9809. DOI:10.1021/ja502692w |
| [25] | X. Feng, L. Chen, Y. Honsho, et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor-acceptor ordering. Adv. Mater. 24 (2012) 3026–3031. DOI:10.1002/adma.v24.22 |
| [26] | M. Calik, F. Auras, L.M. Salonen, et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 136 (2014) 17802–17807. DOI:10.1021/ja509551m |
| [27] | S.B. Jin, K. Furukawa, M. Addicoat, et al. Large pore donor-acceptor covalent organic frameworks. Chem. Sci. 4 (2013) 4505–4511. DOI:10.1039/c3sc52034j |
| [28] | (a) S.B. Jin, M. Supur, M. Addicoat, et al., Creation of superheterojunction polymers viadirect polycondensation: segregated andbicontinuousdonor-acceptor (-columnar arrays in covalent organic frameworks for long-lived charge separation, J. Am. Chem. Soc. 137(2015) 7817-7827; (b) S.B. Jin, X.S. Ding, X. Feng, et al., Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions, Angew. Chem. Int. Ed. 52(2013) 2017-2021. |
| [29] | X.S. Ding, L. Chen, Y. Honsho, et al. An n-channel two-dimensional covalent organic framework. J. Am. Chem. Soc. 133 (2011) 14510–14513. DOI:10.1021/ja2052396 |
| [30] | X. Feng, L.L. Liu, Y. Honsho, et al. High-rate charge-carrier transport in porphyrin covalent organic frameworks: switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 51 (2012) 2618–2622. DOI:10.1002/anie.201106203 |
| [31] | X.K. Gao, Y.B. Hu. Development of n-type organic semiconductors for thin film transistors: a viewpoint of molecular design. J. Mater. Chem. C 2 (2014) 3099–3117. DOI:10.1039/c3tc32046d |
| [32] | (a) J.W. Colson, A.R. Woll, A. Mukherjee, et al., Oriented 2D covalent organic framework thin films on single-layer graphene, Science 332(2011) 228-231; (b) X.H. Liu, C.Z. Guan, S.Y. Ding, et al., On-surface synthesis of singlelayered two-dimensional covalent organic frameworks via solid-vapor interface reactions, J. Am. Chem. Soc. 135(2013) 10470-10474; (c) C.Z. Guan, D. Wang, L.J. Wan, Construction and repair of highly ordered 2D covalent networks by chemical equilibrium regulation, Chem. Commun. 48(2012) 2943-2945; (d) J.I. Feldblyum, C.H. McCreery, S.C. Andrews, et al., Few-layer, large-area, 2D covalent organic framework semiconductor thin films, Chem. Commun. 51(2015) 13894-13897; (e) L.R. Xu, X. Zhou, Y.X. Yu, et al., Surface-confined crystalline two-dimensional covalent organic frameworks via on-surface schiff-base coupling, ACS Nano. 7(2013) 8066-8073. |
| [33] | (a) N.L. Campbell, R. Clowes, L.K. Ritchie, A.I. Cooper, Rapid microwave synthesis and purification of porous covalent organic frameworks, Chem. Mater. 21(2009) 204-206; (b) B.P. Biswal, S. Chandra, S. Kandambeth, et al., Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks, J. Am. Chem. Soc. 135(2013) 5328-5331. |
 2016, Vol. 27
2016, Vol. 27 


