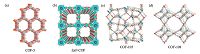
Covalent organic frameworks (COFs) are crystalline organic porous materials connected by strong covalent bonds. Since the first examples of COFs reported by Yaghi et al. in 2005 [1], the research of COFs has evoked an immense amount of recent interest and progressed significantly [2]. Generally, according to the dimensions of their structural features, COFs can be divided into two-dimensional (2D) and three-dimensional (3D) frameworks. 2D COFs usually consist of either single layer structure or multilayer stacking structure assembled with the aid of π-π interactions (e.g. COF-5 and ZnP-COF, Fig. 1a and Fig. b). The layered structures with periodic and infinite topology are formed by covalently linking of multi-functional building blocks. 3D COFs generally expand the network framework to three-dimensional space through tetrahe- drally-structured building blocks (e.g. COF-105 and COF-108, Fig. 1c and d).
|
Download:
|
| Figure 1. Schematic representation of 2D and 3D COFs. Reproduced with the permissions of AAAS from Ref. [1, 2a], and Wiley from Ref. [14]. | |
Compared with other crystalline porous materials, COFs with high surface area, adjustable pore size and tunable internal chemical environment possess many unique advantages: (1) unlike metal-organic frameworks or coordination polymers [3], COFs are usually composed of only light elements, such as B, C, H, N and O, resulting in low mass densities of COFs (e.g. 0.17 g cm-3 for COF-108, 0.18 g cm-3 for COF-105 [1, 2a]); (2) diversities of organic building blocks and synthetic organic reactions facilitate to design and synthesize COFs with specific structures and functions; (3) COFs are constructed from organic building units linked by robust covalent bonds, endowing COFs with high thermal stability, and except for boron linked COFs, most of COFs also have good chemical stability; (4) easy to introduce additional functional groups by post-synthetic modification; (5) possessing highly ordered periodic structures, not only beneficial to study the specific physical and chemical behavior from atomic and molecular level, but also helpful to improve the performance. These advantages make COFs as promising candidates for gas storage and separation[2i,4] , catalysis [5], optoelectronics[2e,6] and energy storage [7] applications. In recent years, some pioneering works have also explored their potential applications in drug delivery [8], water capture [9], trypsin immobilization [10] and high-resolution chromatographic separation [11].
The research in the COFs can be roughly classified into structural-oriented and functional-oriented protocols. For the structural design, researchers often focus on creating crystalline porous materials with the different topologies, arrangements of building units and pore shapes and sizes using reticular chemistry by changing the geometry of building blocks and the symmetry of reactive groups, combined with new synthetic methodologies. For the functional-oriented protocol, the strategies include the design of functional building units, post-synthetic modification, and the encapsulation ofparticular small molecules into the pores. There is a very close structure-function relationship in COFs, varying structural features always result in distinct performance, and imparting COFs with special functions requires intentionally design of structures. The structural features, synthetic reactions, and formation mechanisms of COFs and their applications in gas storage and separation have been well summarized in several pioneering reviews [2b, 2c, 2g-i]. In this mini review, we focus on the recent advances of COFs in electronic and optical applications (Scheme 1) and hope this work could promote further development for COF-based optical-electronic systems.

|
Download:
|
| Scheme. 1. Schematic illustration of COFs' applications in electronic and optical fields. | |
2. Electronic and optical properties 2.1. Semiconduction and photoconduction
In 2D COFs, periodic polymer sheets with different topologies, such as hexagonal or tetragonal geometric shapes, are usually constructed from aromatic building blocks through strong covalent bonding, and the extensive π-π interaction between these sheets drives to form layered stacking structures. Aromatic building units are self-sorted to constitute periodic and vertical columnar arrays that are favored for charge carrier and photoexcited exciton transportation, endowing COFs with semiconductive and photoconductive behaviors. Moreover, simultaneously incorporating donor and acceptor into one framework or introducing one component into the framework and another into the pores offer great opportunities for photoexcited charge transfer and ambipolar transportation capability, which is of vital importance for photoelectronic applications.
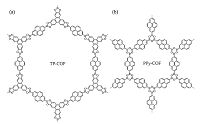
Jiang and co-workers pioneered in this area and firstly reported semiconductive and photoconductive COFs, TP-COF [12] and PPy- COF [13], via co-condensation or self-condensation of highly ordered π-conjugated pyrene derivative. TP-COF (Fig. 2a) shows semiconducting characters and strong blue light emission ability, while PPy-COF (Fig. 2b) is able to harvest visible light and generates prominent photocurrent upon light irradiation.
|
Download:
|
| Figure 2. Schematic representation of TP-COF and PPy-COF. | |
To increase visible and near-infrared light absorption ability, π- electronic macrocycles, such as porphyrin and phthalocyanine, have been incorporated into the framework and extensively investigated.
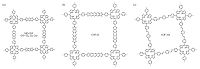
Porphyrins, a typical and large π system with 18 electrons, are both ubiquitous in nature and promising for man-made photon- driven devices. We, Jiang and Irle et al. have prepared three 2D porphyrin COFs with different central metals (MP-COFs, M = H2, Zn, Cu, Fig. 3a) and studied their charge carrier transportation properties by using flash-photolysis time-resolved microwave conductivity (FP/TRMC) method [14]. As a result, the metal ions in porphyrins play a vital role both in forming the metal-on-metal channel for electron conduction and in tuning the carrier mobility. The free-base porphyrin H2P-COF exhibits a high-rate hole transport ability, while CuP-COF displays electron transport capability. Notably, ZnP-COF is the first example that shows ambipolar charge transport ability. Porphyrin-based boronate- ester linked COF-66 (Fig. 3b) and imine-linked COF-366 (Fig. 3c) have been synthesized by Yaghi and co-workers[6f]. Both COF-66 and COF-366 are hole-conducting with charge mobility values of 3.0 and 8.1 cm2 V-1s-1, respectively, indicating that the higher charge carrier mobility is correlated with the eclipsed stacking layers and p-conjugated intralayer structures. Recently, 2D imine-linked porphyrin COFs having tunable component of intralayer H-bonding docking sites were synthesized[6c]. The H-bonding interactions lead to a more planar conformation, endowing the COFs with extended π-cloud delocalization, increased visible photon harvesting capability and reduced band gap, as well as enhanced photocatalytic activity to generate singlet oxygen.
|
Download:
|
| Figure 3. Schematic representation of MP-COF (M = H2, Zn, Cu), COF-66 and COF-366. | |
NiPc-COF (Fig. 4a) shows increased light-harvesting capability in the deep-red visible and near-infrared regions and exhibits hole carrier mobility as high as 1.3 cm2 V-1 s-1[6a]. Meanwhile, another three metallophthalocyanine COFs, MPc COFs (M = Co, Cu and Zn), have also been studied [15]. As a result, the central metal ions of metallophthalocyanine units significantly affect the charge carrier transport behavior and the absorption of light both in the intensity and broadness. Interestingly, the first n-type semiconducting 2D-NiPc-BTDA-COF (Fig. 4b) was obtained by using electron-withdrawing benzothiadiazole (BTDA) as building block to substitute the benzene at the edges of the NiPc-COF. It exhibits completely different charge carrier-transport mode compared to NiPc-COF with p-type characteristics[6b]. 2D- NiPc-BTDA-COF displays an outstanding electron mobility of 0.6 cm2 V-1 s-1, and very broad absorbance up to 1000 nm.

|
Download:
|
| Figure 4. Schematic representation of NiPc COF and 2D-NiPc-BTDA COF. | |
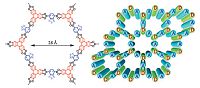
Highly aligned electron donor (D) and acceptor (A) systems with macroscopic heterojunctions are crucial to develop organic electronics. We and Jiang et al. have reported a 2D D-A COF with segregated donor-acceptor alignments (Fig. 5) [16]. As a result, the 2D D-A COF is ambipolar with hole and electron mobilities of 0.01 and 0.04 cm2 V-1s-1, respectively, and large on/off ratio photoconductive responsibility, indicating that the holes and electrons can be separately conducted along the D-on-D and A-on- A columns.
|
Download:
|
| Figure 5. Schematic representation of 2D D-A COF with self-sorted and periodic electron donor–accepter ordering and bicontinuous conducting channels (left: structure of one hexagon; right: a 3 × 3 grid). Reproduced with the permissions of Wiley from Ref. [16]. | |
Jiang, Irle and Fukuzumi et al. have also prepared a series of D-A COFs by using phthalocyanine or triphenylene (TP) acting as donors and diimide derivatives (including pyrommellitic diimide (PyrDI), naphthalene diimide (NDI) and perylene diimide (PDI)) as the acceptors, and systematically investigated the photochemical processes of free charges in these COFs[6e,17] . In DZnPc-ANDI- COF, the D-A heterojunctions enable an ultrafast charge separation and exceptional long-term charge retention[17a]. Interestingly, DTP-APyrDI-COF forms a charge transfer complex, while in contrast, with the similar hexagonal lattice structures, the donor and acceptor columns of DTP-ANDI-COF are independent, leading to a neutral system at the ground state and ready for charge separation[17b]. Notably, in the DMPc-ADI-COFs, each metal-lophthalocyanine donor and diimide acceptor blocks are separately face-to-face stacked to create segregated and bicontinuous D-A p-columnar arrays, thus giving rise to the generation of superheterojunctions to achieve photo-induced electron transfer and long-lived charge separation[6e].
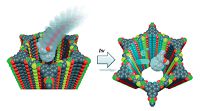
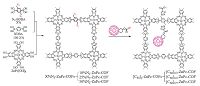
Following this line, another strategy has been established by filling spatially confining electron acceptors in the one-dimensional open channels of electron-donating COFs to prepare donor- acceptor COFs. For example, a thienothiophene-based TT-COF with high surface area of 1800 m2 g-1 and 3 nm open pore system was synthesized via co-condensation of thienothiophene diboronic acid and hexahydroxytriphenylene (HHTP), and it is able to serve as electron donor[6g]. This open framework enables the uptake of fullerene (PCBM) electron acceptor by physical filling, thus forming a novel periodic interpenetrated donor-acceptor system. In the PCBM:TT-COF system, the photoinduced charges can transfer from the TT-COF donor network toward the encapsulated PCBM phase (Fig. 6). This study indicates that light-induced charge-separation and collection is feasible within these systems and this kind of D-A COFs can act as a candidate for photovoltaic cells. In addition, another method for converting the open structures of electron- donating COFs into ordered donor-acceptor heterojunctions has been reported by Jiang and co-workers[6d]. In detail, the electron- donating X%N3-ZnPc-COFs were formed by the three-component condensation of (ZnPc[OH]8), 1, -phenylenediboronic acid (BDBA) and N3-BDBA at different molar ratios to generate ZnPc-COFs containing different contents of -N3 groups on the channel walls. And then via click reaction with electron acceptor 1,2 -(4'-propiolyloxycyclohexno) fullerene, fullerene moieties were immobilized on the channel walls, achieving photoelectric COFs with segregated donor-acceptor alignments (Fig. 7). As a result, the donor-acceptor heterojunctions plays a critical key in photoinduced electron transfer and charge separation, whereas the content of fullerene acceptor affects charge separation efficiency, the more the fullerene molecules, the higher the efficiency of charge separation.
|
Download:
|
| Figure 6. Schematic representation of the host-guest complex of TT-COF and a PCBM molecule (C gray, O red, B green, and S yellow). Reproduced with the permissions of Wiley from Ref.[6g]. | |
|
Download:
|
| Figure 7. Schematic representation of converting open lattice structures into segregated donor-acceptor arrays. | |
For practical applications in photoelectronic devices, thin films are highly desired. Therefore, many groups have paid their attention to prepare COFs-based thin films and investigate their electronic behaviors. Guo and Jiang et al. reported a p-conjugated organic framework (CS-COF) with inborn periodic ordering of conjugated chains by co-condensation of triphenylene hexamine and tert-butylpyrene tetraone [18]. They fabricated optoelectronic devices with a configuration of Al/poly(methyl methacrylate) (as a glue):CS-COF ⊃ C60/Au, and the device exhibits photoresponsive ability. The relatively lower power conversion efficiency (0.9%) of the device is probably attributed to the random orientation of the COFs in the film. Cai, Zhang and Liu et al. reported imine-based TTF- COF oriented thin films fabricated by in situ growing on various substrates (ITO glass, silicon wafer with or without pre-patterned Au electrodes) [19]. The conductivity of TTF-COF films was measured to be 1.2 × 10-4Sm-1. It should be noted that upon doping the films with I2 or TCNQ for redox reactions, the conductivity was boosted to 0.28 S m-1. In addition, an electron- donor COF (BDT-COF) based on a benzodithiophene core was synthesized as thin film on different polycrystalline surfaces, and fullerene derivatives as electron-acceptor were impregnated into these thin films to obtain mixed electron-donor/acceptor host- guest materials. The results show that efficient light-induced charge transfer from the BDT-framework to fullerene is occurred [20]. Notably, large-area imine-based COF thin films have been fabricated at the solution/air interface, and the obtained films could be picked up and transferred to different substrates. Thus obtained films could be applied as semiconducting active layers in field-effect transistors [21]. Li, Tian and Tang et al. have prepared photoconductive COF films by in situ growth method. They showed that compared with randomly oriented films, oriented film exhibits improved photoresponse speed and higher charge transportation ability [22].
To sum up, face-to-face stacked p-aromatic building units in 2D COFs can form efficient charge transport path, while conjugated COFs, such as imine-based COFs, provide another transportation path along the conjugated planar polygons. Changing the electron densities of framework either by varying the building blocks or introducing donor/accepter species into the pores offers effective ways to alter and control their semiconductive and photoconductive performance. Crystalline 2D COFs provides a useful platform for the study of charge transfer and separation, and the progress aiming to fabricate large-area and non-defective thin films will promote the real applications of 2D COFs in optoelectronic devices in the future.
2.2. Energy storageCOFs with well-defined and easy-modified pore environment facilitate the incorporation of electronic-active moieties and the transportation of electrolyte ions, making them serve as promising candidate materials for energy storage and conversion.
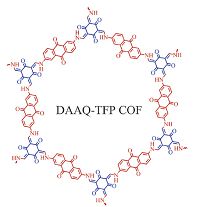
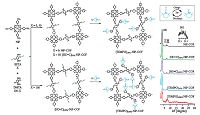
The first COF (termed DAAQ-TFP COF (Fig. 8)) that is capable of reversible Faradaic process was reported by Dichtel and coworkers[7b]. DAAQ-TFP COF was synthesized by condensing the redox-active 2,6 -diaminoanthraquinone (DAAQ) with 1, 3, 5 -trifor-mylphloroglucinol (TFP), and it exhibits reversible electrochemical processes and remarkable stability to a strongly acidic electrolyte. The DAAQ-TFP COF modified electrodes possess increased capacitance compared with DAAQ (electroacitve monomer) and a COF lacking redox-active groups, and its capacitance can be maintained even after 5000 charge-discharge cycles. They further prepared oriented thin film of DAAQ-TFP COF through the slow introduction of the monomer into a solution containing Au substrates. Compared with the randomly oriented COF slurry (0.4 mF cm-2), the oriented COF film shows a much higher areal capacitance (3 mF cm-2), owing to the improved accessibility of their redox- active moieties [23]. Recently, a facile and general post-synthetic modification strategy has been proposed for transforming a conventional COF to an outstanding redox-active platform for energy storage[7a]. This is the first example of radical COFs prepared through post-synthetic channel-wall functionalization with organic radicals (Fig. 9). As a result, two radical COFs exhibit muchhighercapacitance(167 and 124 F g-1for[TEMPO]100%-NiP-COF and [TEMPO]50%-NiP-COF, respectively) than that of redox- active DAAQ-TFP COF (40 F g-1), and display high-rate electrode kinetics and outstanding cycle performance. In addition, COFs/ graphene composite as supercapacitor electrodes was synthesized and shows a specific capacitance of 533 F g -1 at 0.2 A g-1 [24].
|
Download:
|
| Figure 8. Schematic representation of redox-active DAAQ-TFP COF. | |
|
Download:
|
| Figure 9. (a) Syntheses of radical COFs ([TEMPO]100%-NiP-COF and [TEMPO]50%-NiP-COF) with TEMPO radicals immobilized on the walls by functionalization of [HC≡C]100%-NiPCOF and [HC≡C]50%-NiP-COF. (b) The charge-discharge process of TEMPO based on a one-electron redox reaction. (c) XRD patterns of the COFs. Reproduced with the permissions of Wiley from Ref.[7a]. | |
In addition, a redox-active COF (DTP-ANDI-COF) on conductive carbon nanotubes (CNTs) prepared by in situ polycondensation was reported using as cathode electrode for energy storage in lithium-ion batteries [25]. The resulting DTP-ANDI-COF@CNTs cathode exhibits robust cycle stability and high-rate capability. The capacity of the DTP-ANDI-COF@CNTs battery could be retained even after 700 cycles at 2.4 C with high Coulombic efficiency.
COFs can also serve as potential host materials for sulfur impregnation in order to improve the performance of lithium- sulfur batteries. Such as CTF-1 [26] and Azo-COF [27] have been reported as the host for sulfur in Li-S batteries. The composite cathodes of COFs with sulfur were prepared by a melt-diffusion strategy. The capacity of CTF-1/S@155 ℃ cathode is maintained at 762 mA h g-1 after 50 cycles at 0.1 C, while S/Azo-COF cathode presents a high capacity of 741 mA h g-1 after 100 cycles at 0.1 C. Furthermore, S-CTF-1 with embedded polymeric sulfur was synthesized in situ from 1, -dicyanbenzen and elemental sulfur in the absence of catalysts and solvents, resulting covalent attachment of sulfur on the pore walls with high sulfur loadings up to 62 wt% [28]. S-CTF-1 as a sulfur cathode shows high cycling performance in Li-S batteries with 85.8% capacity retention after 300 cycles at 1 C and superior initial Coulombic efficiency (Fig. 10).
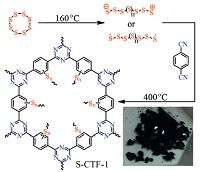
|
Download:
|
| Figure 10. Synthesis of S-CTF-1 from elemental sulfur and an optical image of S-CTF-1. Reproduced with the permissions of Wiley from Ref. [28]. | |
The design of highly mobile ions in crystalline solids is desired for applications such as battery electrolytes. Lee and Zhang et al. have reported ionic COFs, ICOF-1 and ICOF-2, which contain spiroborate linkages with sp3 hybridized boron anionic centers and tunable countercations (Fig. 11) [29]. ICOF-2 doping with Li+ as counterions exhibits solid electrolytes characters and remarkable Li+ transference number with the value of 0.80 ± 0.02, which is higher than typical solid-state polymer electrolytes doping with lithium salt. These highly stable ionic COFs show great potential to be solid lithium electrolytes/separators.
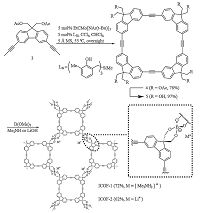
|
Download:
|
| Figure 11. Syntheses of ICOF-1 and ICOF-2. Reproduced with the permissions of Wiley from Ref. [29]. | |
The development of proton conduction membranes with better performance appears to be at the heart of the fuel-cell technology. The proton conducting COF material has been reported for the first time by Banerjee and co-workers[6h]. The material was prepared by immobilizing H3PO4 into an azo functionalized COF (Tp-Azo, Fig. 12) and enables proton conduction within it. The resulting PA@Tp-Azo displays decent proton conductivity under both hydrous (9.9 × 10-4 S cm-1) and anhydrous conditions (6.7 × 10-5 S cm-1). Whereas, if the Tp-Azo COF was substituted by stilbene constructed COF (Tp-Stb), thus-obtained material (PA@Tp-Stb) only exhibits poor proton conductivity under hydrous conditions. These results demonstrate that the proton responsive azo group plays a crucial role in facilitating proton conduction. Recently, Kundu, Kurungot and Banerjee et al. prepared H3PO4 loaded COFs (PA@TpBpy-MC) by mechanochemical method, and it exhibits remarkable proton conductivity (2.5 × -3 S cm-1 at 120 ℃) and solid-state electrolyte conductivity (1.4 × 10 -2 S cm-1),which is superior to PA@TpBpy-ST synthesized by solvothermal method under the similar conditions [30]. In addition, Kundu and Banerjee et al. also synthesized a both sulfonic acid- and pyridine- functionalized COF, TpPa-(SO3H-Py), followed by loading phytic acid into the pores. The resultant phytic@TpPa-(SO3H-Py) possesses intrinsic and extrinsic proton conducting ability (Fig. 13) [31]. Jiang and co-workers loaded N-heterocyclic triazole and imidazole as organic proton carriers into the 1D channels of mesoporous TPB- DMTP-COF to prepare trz@TPB-DMTP-COF and im@TPB-DMTP- COF with high loading amounts of 180 wt% and 155 wt%, respectively. These two materials exhibit proton conductivities of 1.1 × 10-3 S cm-1 and 4.37 × 10-3 S cm-1 at 130 ℃, respectively [32]. The following control experiments were conduced to investigate the influence factors of proton conduction: using deuterium-substituted imidazole (d4-im) as the proton carrier; decreasing the content of imidazole embedded in the pores; loading an inactive molecule inside the channels and covering the surface the COFs with the mixture of the inactive molecule and imidazole; employing an TPB-TP-COF with low crystallinity and porosity. As a result, all of these structural factors of COFs, including the skeletons, pore shape, pore environment and pore density, play a vital role in proton conducting. Li and Zang et al. have reported the first cationic COF with Br- as counterions, termed EB-COF:Br, and via counterion exchange processes, the other four charged COFs (EB-COF:X, X = F, Cl, I, PW12O403- (abbreviate as PW12)) have been prepared [33]. The results demonstrate that the porosities and pore sizes of EB-COFs could be finely modulated through facile anionic-exchange processes. Notably, EB-COF:PWi2 exhibits the best proton conductivity at room temperature (3.32 × 10-3S cm-1 at 97% RH) of the porous organic materials reported to date.
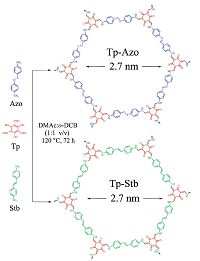
|
Download:
|
| Figure 12. Schematic of Tp-Azo and Tp-Stb syntheses. | |
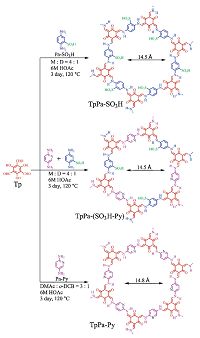
|
Download:
|
| Figure 13. Schematic representation of the synthesis of TpPa-SO3H, TpPa-(SO3H-Py), and TpPa-Py with their hexagonal pores. | |
Generally in the COF systems, redox-active moieties regulate energy storage and release, while the open channels govern the transport of ions. To improve the performance, highly oriented arrangement of COFs and/or incorporating with conductive species is needed to ensure the required conductivity and provide efficient charge/proton transportation pathway.
2.3. ChemosensorCOFs takes several advantages as optical chemosensors, such as (1) high porosity, tunable pore structure and environment, and large surface area enable the pre-enrichment of guest materials to increase sensitivity; (2) linkers that utilized to construct COFs are usually rigid π-conjugated aromatic arenes, making COFs highly luminescent; (3) the diversities of linker choices and the ease of postsynthetic modification are benefit to improve the interactions between host and guest materials and thus increasing the selectivity.
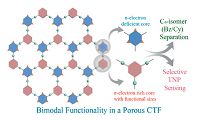
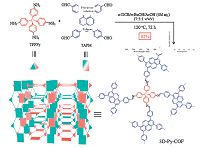
An azine-linked pyrene COF has been used as a chemosensor for detecting explosive molecules [34]. The Py-Azine COF with the extended π conjugation skeleton is highly luminescent, whereas the azine fragments provide docking sites to lock guest molecules. As a result, the Py-Azine COF shows an outstanding sensitivity and selectivity in response to trinitrophenol (TNP) type explosive. Ghosh et al. has reported a bimodal functionalized triazine-based framework named CTF-IP10 (Fig. 14) [35]. On the one hand, electron-deficient triazine rings benefit to selective uptake benzene with the value of 12.43 mol kg-1 and separate it from its saturated cyclic congener, cyclohexane. On the other hand, having Lewis basic sites within the framework, electron-rich aromatic rings can interact with TNP by intermolecular hydrogen bonding and result in fluorescence quenching. In addition, thinlayered TfpBDH-CONs were prepared by liquid phase exfoliation method, exhibiting increased luminescence intensity compared with their bulk COFs [36]. Notably, the TfpBDH-CONs displays highly selective and visual detection ability for TNP in the solid state. Qiu and Yan et al. reported that the dye-doped PI-COF-3 shows sensitive temperature-dependent photoluminescence behavior [37], and Zheng have showed that TAT-COFs can realize turn-on detection of electron rich arenes and turn-off response to the electron deficient arenes [38]. Wang's group has reported that fluorescent thioether-functionalized COF-LZU8 is available to both detect and facilely remove toxic Hg2+ [39], and Liu et al. have synthesized COF-JLU3 used as a fluorescent sensor for Cu2+ [40]. Moreover, a fluorescent 3D-Py-COF (Fig. 15) was first reported by Wang and co-workers [41]. It should be noted that in the 3D COFs, the π-π stacking is avoided and thus the fluorescent quantum yield can be efficiently improved. The 3D-Py- COF exhibits yellow-green luminescence, and the fluorescence will be quenched if adding picric acid to its suspension in DMF. Recently, Jiang et al. have prepared a AIE-based COF by introducing AIE-active tetraphenylethene (TPE) as building unit [42]. The obtained TPE-Ph COF shows the highest luminescence quantum yield among the reported COFs and can be used for the detection of specific chemicals, such as ammonia, as highly sensitive sensor.
|
Download:
|
| Figure 14. Representation of the bimodal functionality in CTF-IP10 by virtue of the electron-deficient and electron-rich pore surface. Reproduced with the permissions of Wiley from Ref. [35]. | |
|
Download:
|
| Figure 15. Schematic representation of the synthesis and extended two-fold interpenetrated structure of 3D-Py-COF. Reproduced with the permissions of American Chemical Society from Ref. [41]. | |
In addition, a photoresponsive structurally dynamic COF, Ph- An-COF, with the anthracene building blocks as photo-responsive units, has been firstly reported by Jiang and co-workers [43]. Irradiation of Ph-An-COF causes interlayer [4π + 4π] cycloaddition reactions accompanied by changes in properties and functions, and such photoinduced hierarchical transformations are thermally reversible.
3. Summary and perspectivesIn conclusion, COFs, as an emerging class of crystalline porous materials, have been developed rapidly in the past 10 years. Because of high surface areas, permanent porosity, regular arrangement of porous channels and tunable inner environment of cavities, COFs have shown numerous potential applications in different fields. So far, researchers have done lots of work on development of synthetic methodology, design of novel structures, exploration of new properties, and fabrication of COFs into devices, however, there are still many challenges to be overcome.
For COF materials, it is hard to form single crystals, and the real structures of COFs as well as the layer-stacking sequences (staggered or eclipsed) are still controversial although they can be simulated based on the data of powder X-ray diffraction (PXRD), hence getting single crystals and characterization by advanced technologies are the challenges for researchers in this field. Furthermore, the formation reactions of crystalline and ordered COFs are mainly limited to reversible reactions of forming boric acid ester and imine double bonds, giving rise to the disadvantage of poor chemical stability. Therefore, exploring novel reactions and connection methods in order to synthesize COFs with both longrange order and enhanced chemical stability is another important aspect to be explored. In addition, further study on the structure- performance relationship via the design of skeleton structures, functionalization of organic building blocks and introduction of guest molecules and exploring the key factors involving in functional control are still required to achieve highly functionalized materials.
On the other hand, it is difficult to fabricate COFs into devices by the reason of their inmeltable and insoluble nature. Hence, to develop facile and versatile strategies for processing COF into thin films and shaped bodies is of vital importance in practical applications [2f-h]. To date, the synthesized COF films are mainly focus on the supporting membrane adopting on-surface synthesis. One strategy is in situ synthesis on different substrates, such as Ag(111) surface [44], single-layer graphene (SLG) [45] and highly oriented pyrolytic graphite (HOPG) [46]. However, it is difficult to control the dense, thickness and uniformity of the oriented films, and most importantly, large-areal and stand-alone films are difficult to be obtained. Another method is to exfoliate layered COFs into few layers, and then followed by depositing them onto a surface [47]. This method is still hard to obtain larger-scale and non-defective films. Recently, some new synthetic methods for COF films have been reported, such as solid-vapor interface reactions [48], solution/solid interface reactions [49], air/water interface reactions (Langmuir-Blodgett method) [50], photolithography and reactive ion etching techniques [51], and the effect of monomer structure of isomeric single-layered COFs on the growth kinetics process has also been investigated [52]. These studies provide basis for the preparation of larger-scale and high- quality COF thin films. The processing of COFs into shaped bodies is still a vast land to be explored. This intriguing aspect is worthy of further study to make COFs into real-world applications.
In a word, COFs provides a new platform for design and synthesis of novel functional organic porous materials and have broad application prospects. Despite the difficulties to be overcome to achieve commercial applications, we anticipate a bright future for COFs as a promising platform to solve environmental issues.
Acknowledgment We are grateful to the 973 Program (No. 2013CB834704), the National Natural Science Foundation of China (Nos. 21471018, 21201018, 21404010) and 1000 Plan (Youth) for financial support.| [1] | A.P. Côte, A.I. Benin, N.W. Ockwig, et al. Porous, crystalline, covalent organic frameworks. Science 310 (2005) 1166–1170. DOI:10.1126/science.1120411 |
| [2] | (a) H.M. El-Kaderi, J.R. Hunt, J.L. Mendoza-Cortés, et al., Designed synthesis of 3D covalent organic frameworks, Science 316(2007) 268-272; (b) X. Feng, X.S. Ding, D.L. Jiang, Covalent organic frameworks, Chem. Soc. Rev. 41(2012) 6010-6022; (c) S.Y. Ding, W. Wang, Covalent organic frameworks (COFs): from design to applications, Chem. Soc. Rev. 42(2013) 548-568; (d) J.W. Colson, W.R. Dichtel, Rationally synthesized two-dimensional polymers, Nat. Chem. 5(2013) 453-465; (e) M. Dogru, T. Bein, On the road towards electroactive covalent organic frameworks, Chem. Commun. 50(2014) 5531-5546; (f) X.H. Liu, C.Z. Guan, D. Wang, L.J. Wan, Graphene-like single-layered covalent organic frameworks: synthesis strategies and application prospects, Adv. Mater. 26(2014) 6912-6920; (g) P.J. Waller, F. Gándara, O.M. Yaghi, Chemistry of covalent organic frameworks, Acc. Chem. Res. 48(2015) 3053-3063; (h) U. Díaz, A. Corma, Ordered covalent organic frameworks, COFs and PAFs. From preparation to application, Coordin. Chem. Rev. 311(2016) 85-124; (i) Y.F. Zeng, R.Q. Zou, Y.L. Zhao, Covalent organic frameworks for CO2 capture, Adv. Mater. 28(2016) 2855-2873. |
| [3] | (a) H. Furukawa, K.E. Cordova, M. O'Keeffe, O.M. Yaghi, The chemistry and applications of metal-organic frameworks, Science 341(2013) 1230444; (b) Y. Bai, Y.B. Dou, L.H. Xie, et al., Zr-based metal-organic frameworks: design, synthesis, structure, and applications, Chem. Soc. Rev. 45(2016) 2327-2367; (c) B. Wang, H. Yang, Y.B. Xie, et al., Controlling structural topology of metalorganic frameworks with a desymmetric 4-connected ligand through the design of metal-containing nodes, Chin. Chem. Lett. 27(2016) 502-506; (d) S. Kitagawa, R. Kitaura, S.I. Noro, Functional porous coordination polymers, Angew. Chem. Int. Ed. 43(2004) 2334-2375; (e) S. Kitagawa, K. Uemura, Dynamic porous properties of coordination polymers inspired by hydrogen bonds, Chem. Soc. Rev. 34(2005) 109; (f) D. Tian, X.J. Liu, R.Y. Chen, Y.H. Zhang, Syntheses, structures, luminescent and magnetic properties of two coordination polymers based on a flexible multidentate carboxylate ligand, Chin. Chem. Lett. 26(2015) 499-503. |
| [4] | (a) N. Huang, X. Chen, R. Krishna, D.L. Jiang, Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization, Angew. Chem. Int. Ed. 54(2015) 2986-2990; (b) N. Huang, R. Krishna, D.L. Jiang, Tailor-made pore surface engineering in covalent organic frameworks: systematic functionalization forperformance screening, J. Am. Chem. Soc. 137(2015) 7079-7082; (c) C.J. Doonan, D.J. Tranchemontagne, T.G. Glover, J.R. Hunt, O.M. Yaghi, Exceptional ammonia uptake by a covalent organic framework, Nat. Chem. 2(2010) 235-238; (d) H. Furukawa, O.M. Yaghi, Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications, J. Am. Chem. Soc. 131(2009) 8875-8883; (e) J.R. Song, J.L. Sun, J.M. Liu, Z.T. Huang, Q.Y. Zheng, Thermally/hydrolytically stable covalent organic frameworks from a rigid macrocyclic host, Chem. Commun. 50(2014) 788-791; (f) Z.P. Li, X. Feng, Y.C. Zou, et al., A 2D azine-linked covalent organic framework for gas storage applications, Chem. Commun. 50(2014) 13825-13828; (g) Z.P. Li, Y.F. Zhi, X. Feng, et al., An azine-linked covalent organic framework: synthesis, characterization and efficient gas storage, Chem. Eur. J. 21(2015) 12079-12084; (h) H.P. Ma, H. Ren, S. Meng, et al., A 3D microporous covalent organic framework with exceedingly high C3H8/CH4 and C2 hydrocarbon/CH4 selectivity, Chem. Commun. 49(2013) 9773-9775; (i) H. Wei, S.Z. Chai, N.T. Hu, et al., The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity, Chem. Commun. 51(2015) 12178-12181. |
| [5] | (a) S. Lin, C.S. Diercks, Y.B. Zhang, et al., Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water, Science 349(2015) 1208-1213; (b) S.Y. Ding, J. Gao, Q. Wang, et al., Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in suzuki-miyaura coupling reaction, J. Am. Chem. Soc. 133(2011) 19816-19822; (c) P. Pachfule, M.K. Panda, S. Kandambeth, et al., Multifunctional and robust covalent organic framework-nanoparticle hybrids, J. Mater. Chem. A 2(2014) 7944-7952; (d) Y. Wu, H. Xu, X. Chen, J. Gao, D.L. Jiang, A π-electronic covalent organic framework catalyst: π-walls as catalytic beds for diels-alder reactions under ambient conditions, Chem. Commun. 51(2015) 10096-10098; (e) Q.R. Fang, S. Gu, J. Zheng, et al., 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis, Angew. Chem. Int. Ed. 53(2014) 2878-2882; (f) V.S. Vyas, F. Haase, L. Stegbauer, et al., A tunable azine covalent organic framework platform for visible light-induced hydrogen generation, Nat. Commun. 6(2015) 8508; (g) H. Xu, J. Gao, D.L. Jiang, Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts, Nat. Chem. 7(2015) 905-912. |
| [6] | (a) X.S. Ding, J. Guo, X. Feng, et al., Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity, Angew. Chem. Int. Ed. 50(2011) 1289-1293; (b) X.S. Ding, L. Chen, Y. Honsho, et al., An n-channel two-dimensional covalent organic framework, J. Am. Chem. Soc. 133(2011) 14510-14513; (c) X. Chen, M. Addicoat, E.Q. Jin, et al., Locking covalent organic frameworks with hydrogen bonds: general and remarkable effects on crystalline structure, physical properties, and photochemical activity, J. Am. Chem. Soc. 137(2015) 3241-3247; (d) L. Chen, K. Furukawa, J. Gao, et al., Photoelectric covalent organic frameworks: converting open lattices into ordered donor-acceptor heterojunctions, J. Am. Chem. Soc. 136(2014) 9806-9809; (e) S.B. Jin, M. Supur, M. Addicoat, et al., Creation of superheterojunction polymers via direct polycondensation: segregated and bicontinuous donor-acceptor π-columnar arrays in covalent organic frameworks for long-lived charge separation, J. Am. Chem. Soc. 137(2015) 7817-7827; (f) S. Wan, F. Gándara, A. Asano, et al., Covalent organic frameworks with high charge carrier mobility, Chem. Mater. 23(2011) 4094-4097; (g) M. Dogru, M. Handloser, F. Auras, et al., A photoconductive thienothiophenebased covalent organic framework showing charge transfer towards included fullerene, Angew. Chem. Int. Ed. 52(2013) 2920-2924; (h) S. Chandra, T. Kundu, S. Kandambeth, et al., Phosphoric acid loaded Azo (-N5N-) based covalent organic framework for proton conduction, J. Am. Chem. Soc. 136(2014) 6570-6573. |
| [7] | (a) F. Xu, H. Xu, X. Chen, et al., Radical covalent organic frameworks: a general strategy to immobilize open-accessible polyradicals for high-performance capacitive energy storage, Angew. Chem. Int. Ed. 54(2015) 6814-6818; (b) C.R. DeBlase, K.E. Silberstein, T.T. Truong, et al., β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage, J. Am. Chem. Soc. 135(2013) 16821-16824. |
| [8] | Q.R. Fang, J.H. Wang, S. Gu, et al. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 137 (2015) 8352–8355. DOI:10.1021/jacs.5b04147 |
| [9] | B.P. Biswal, S. Kandambeth, S. Chandra, et al. Pore surface engineering in porous, chemically stable covalent organic frameworks for water adsorption. J. Mater. Chem. A 3 (2015) 23664–23669. DOI:10.1039/C5TA07998E |
| [10] | S. Kandambeth, V. Venkatesh, D.B. Shinde, et al. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 6 (2015) 6786. DOI:10.1038/ncomms7786 |
| [11] | C.X. Yang, C. Liu, Y.M. Cao, X.P. Yan. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 51 (2015) 12254–12257. DOI:10.1039/C5CC03413B |
| [12] | S. Wan, J. Guo, J. Kim, H. Ihee, D.L. Jiang. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 47 (2008) 8826–8830. DOI:10.1002/anie.v47:46 |
| [13] | S. Wan, J. Guo, J. Kim, H. Ihee, D.L. Jiang. A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. 48 (2009) 5439–5442. DOI:10.1002/anie.v48:30 |
| [14] | X. Feng, L.L. Liu, Y. Honsho, et al. High-rate charge-carrier transport in porphyrin covalent organic frameworks: switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 51 (2012) 2618–2622. DOI:10.1002/anie.201106203 |
| [15] | X.S. Ding, X. Feng, A. Saeki, et al. Conducting metallophthalocyanine 2D covalent organic frameworks: the role of central metals in controlling π-electronic functions. Chem. Commun. 48 (2012) 8952–8954. DOI:10.1039/c2cc33929c |
| [16] | X. Feng, L. Chen, Y. Honsho, et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor-acceptor ordering. Adv. Mater. 24 (2012) 3026–3031. DOI:10.1002/adma.v24.22 |
| [17] | (a) S.B. Jin, X.S. Ding, X. Feng, et al., Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions, Angew. Chem. Int. Ed. 52(2013) 2017-2021; (b) S.B. Jin, K. Furukawa, M. Addicoat, et al., Large pore donor-acceptor covalent organic frameworks, Chem. Sci. 4(2013) 4505-4511. |
| [18] | J. Guo, Y.H. Xu, S.B. Jin, et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4 (2013) 2736. |
| [19] | S.L. Cai, Y.B. Zhang, A.B. Pun, et al. Tunable electrical conductivity in oriented thin films of tetrathiafulvalene-based covalent organic framework. Chem. Sci. 5 (2014) 4693–4700. DOI:10.1039/C4SC02593H |
| [20] | D.D. Medina, V. Werner, F. Auras, et al. Oriented thin films of a benzodithiophene covalent organic framework. ACS Nano 8 (2014) 4042–4052. DOI:10.1021/nn5000223 |
| [21] | J.I. Feldblyum, C.H. McCreery, S.C. Andrews, et al. Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Commun. 51 (2015) 13894–13897. DOI:10.1039/C5CC04679C |
| [22] | Y. Chen, H.J. Cui, J.Q. Zhang, et al. Surface growth of highly oriented covalent organic framework thin film with enhanced photoresponse speed. RSC Adv. 5 (2015) 92573–92576. DOI:10.1039/C5RA19430J |
| [23] | C.R. DeBlase, K. Hernández-Burgos, K.E. Silberstein, et al. Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 9 (2015) 3178–3183. DOI:10.1021/acsnano.5b00184 |
| [24] | P.Y. Wang, Q. Wu, L.F. Han, et al. Synthesis of conjugated covalent organic frameworks/graphene composite for supercapacitor electrodes. RSC Adv. 5 (2015) 27290–27294. DOI:10.1039/C5RA02251G |
| [25] | F. Xu, S.B. Jin, H. Zhong, et al. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 5 (2015) 8225. DOI:10.1038/srep08225 |
| [26] | H.P. Liao, H.M. Ding, B.J. Li, X.P. Ai, C. Wang. Covalent-organic frameworks: potential host materials for sulfur impregnation in lithium-sulfur batteries. J. Mater. Chem. A 2 (2014) 8854–8858. DOI:10.1039/c4ta00523f |
| [27] | X.F. Yang, B. Dong, H.Z. Zhang, et al. Sulfur impregnated in a mesoporous covalent organic framework for high performance lithium-sulfur batteries. RSC Adv. 5 (2015) 86137–86143. DOI:10.1039/C5RA16235A |
| [28] | S.N. Talapaneni, T.H. Hwang, S.H. Je, et al. Elemental-sulfur-mediated facile synthesis of a covalent triazine framework for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 55 (2016) 3106–3111. DOI:10.1002/anie.201511553 |
| [29] | Y. Du, H. Yang, J.M. Whiteley, et al. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 55 (2016) 1737–1741. DOI:10.1002/anie.201509014 |
| [30] | D.B. Shinde, H.B. Aiyappa, M. Bhadra, et al. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 4 (2016) 2682–2690. DOI:10.1039/C5TA10521H |
| [31] | S. Chandra, T. Kundu, K. Dey, et al. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 28 (2016) 1489–1494. DOI:10.1021/acs.chemmater.5b04947 |
| [32] | H. Xu, S.S. Tao, D.L. Jiang. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. (2016) . |
| [33] | H.P. Ma, B.L. Liu, B. Li, et al. Cationic covalent organic frameworks: a simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 138 (2016) 5897–5903. DOI:10.1021/jacs.5b13490 |
| [34] | S. Dalapati, S.B. Jin, J. Gao, et al. An azine-linked covalent organic framework. J. Am. Chem. Soc. 135 (2013) 17310–17313. DOI:10.1021/ja4103293 |
| [35] | A. Karmakar, A. Kumar, A.K. Chaudhari, et al. Bimodal functionality in a porous covalent triazine framework by rational integration of an electron-rich and -deficient pore surface. Chem. Eur. J. 22 (2016) 4931–4937. DOI:10.1002/chem.v22.14 |
| [36] | G. Das, B.P. Biswal, S. Kandambeth, et al. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 6 (2015) 3931–3939. DOI:10.1039/C5SC00512D |
| [37] | Q.R. Fang, Z.B. Zhuang, S. Gu, et al. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 5 (2014) 4503. |
| [38] | Y.F. Xie, S.Y. Ding, J.M. Liu, W. Wang, Q.Y. Zheng. Triazatruxene based covalent organic framework and its quick-response fluorescence-on nature towards electron rich arenes. J. Mater. Chem. C 3 (2015) 10066–10069. DOI:10.1039/C5TC02256H |
| [39] | S.Y. Ding, M. Dong, Y.W. Wang, et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II). J. Am. Chem. Soc. 138 (2016) 3031–3037. DOI:10.1021/jacs.5b10754 |
| [40] | Z.P. Li, Y.W. Zhang, H. Xia, Y. Mu, X.M. Liu. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 52 (2016) 6613–6616. DOI:10.1039/C6CC01476C |
| [41] | G.Q. Lin, H.M. Ding, D.Q. Yuan, B.S. Wang, C. Wang. A pyrene-based, fluorescent three-dimensional covalent organic framework. J. Am. Chem. Soc. 138 (2016) 3302–3305. DOI:10.1021/jacs.6b00652 |
| [42] | S. Dalapati, E.Q. Jin, M. Addicoat, T. Heine, D.L. Jiang. Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 138 (2016) 5797–5800. DOI:10.1021/jacs.6b02700 |
| [43] | N. Huang, X.S. Ding, J. Kim, H. Ihee, D.L. Jiang. A photoresponsive smart covalent organic framework. Angew. Chem. Int. Ed. 54 (2015) 8704–8707. DOI:10.1002/anie.201503902 |
| [44] | N.A.A. Zwaneveld, R. Pawlak, M. Abel, et al. Organized formation of 2D extended covalent organic frameworks at surfaces. J. Am. Chem. Soc. 130 (2008) 6678–6679. DOI:10.1021/ja800906f |
| [45] | (a) J.W. Colson, A.R. Woll, A. Mukherjee, et al., Oriented 2D covalent organic framework thin films on single-layer graphene, Science 332(2011) 228-231; (b) E.L. Spitler, J.W. Colson, F.J. Uribe-Romo, et al., Lattice expansion of highly oriented 2D phthalocyanine covalent organic framework films, Angew. Chem. Int. Ed. 51(2012) 2623-2627; (c) E.L. Spitler, B.T. Koo, J.L. Novotney, et al., A 2D covalent organic framework with 4.7-nm pores and insight into its interlayer stacking, J. Am. Chem. Soc. 133(2011) 19416-19421; (d) J.W. Colson, J.A. Mann, C.R. DeBlase, W.R. Dichtel, Patterned growth of oriented 2D covalent organic framework thin films on single-layer graphene, J. Polym. Sci. Pol. Chem. 53(2015) 378-384; (e) L.R. Xu, X. Zhou, W.Q. Tian, et al., Surface-confined single-layer covalent organic framework on single-layer graphene grown on copper foil, Angew. Chem. Int. Ed. 53(2014) 9564-9568. |
| [46] | (a) C.Z. Guan, D. Wang, L.J. Wan, Construction and repair of highly ordered 2D covalent networks by chemical equilibrium regulation, Chem. Commun. 48(2012) 2943-2945; (b) W.L. Dong, L. Wang, H.M. Ding, et al., Substrate orientation effect in the onsurface synthesis of tetrathiafulvalene-integrated single-layer covalent organic frameworks, Langmuir 31(2015) 11755-11759. |
| [47] | (a) I. Berlanga, M.L. Ruiz-González, J.M. Gonzaález-Calbet, et al., Delamination of layered covalent organic frameworks, Small 7(2011) 1207-1211; (b) D.N. Bunck, W.R. Dichtel, Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks, J. Am. Chem. Soc. 135(2013) 14952-14955; (c) S. Chandra, S. Kandambeth, B.P. Biswal, et al., Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination, J. Am. Chem. Soc. 135(2013) 17853-17861. |
| [48] | X.H. Liu, C.Z. Guan, S.Y. Ding, et al. On-surface synthesis of single-layered twodimensional covalent organic frameworks via solid-vapor interface reactions. J. Am. Chem. Soc. 135 (2013) 10470–10474. DOI:10.1021/ja403464h |
| [49] | D. Cui, J.M. MacLeod, M. Ebrahimi, D.F. Perepichka, F. Rosei. Solution and air stable host/guest architectures from a single layer covalent organic framework. Chem. Commun. 51 (2015) 16510–16513. DOI:10.1039/C5CC07059G |
| [50] | W.Y. Dai, F. Shao, J. Szczerbiński, et al. Synthesis of a two-dimensional covalent organic monolayer through dynamic imine chemistry at the air/water interface. Angew. Chem. Int. Ed. 55 (2016) 213–217. DOI:10.1002/anie.201508473 |
| [51] | X.H. Gou, Q. Zhang, Y.L. Wu, et al. Preparation and engineering of oriented 2D covalent organic framework thin films. RSC Adv. 6 (2016) 39198–39203. DOI:10.1039/C6RA07417K |
| [52] | X.H. Liu, Y.P. Mo, J.Y. Yue, et al. Isomeric routes to schiff-base single-layered covalent organic frameworks. Small 10 (2014) 4934–4939. DOI:10.1002/smll.v10.23 |
 2016, Vol. 27
2016, Vol. 27 


