Covalent organic frameworks (COFs) represent an emerging class of porous crystalline polymers composed of light-weight elements and constructed via strong covalent bonds [1-3]. Due to their permanent porosity, structural periodicity and high thermal stability, COFs have triggered substantial research interest over the past decade for potential applications in gas storage and separation [4-9], catalysis [10, 11], sensing [12, 13], optoelectronic devices [14, 15] and energy storage [16-18]. Two-dimensional (2D) COFs, in which the 2D polymer sheets stack in a columnar manner to form a layered eclipsed structure [19], provide a unique means to construct ordered p systems for efficient charge carrier transport through the framework and have potential for developing new π- electronic and photofunctional materials [20]. Therefore, once incorporating certain functional π-electron systems into the frameworks, the resulting 2D COFs are expected to possess unique optical and electrical properties [14]. Various photoelectric units, such as thiophene [21] and tetrathiafulvalene [22, 23], have been successfully developed for 2D COFs and the resulting materials have shown interesting application in optoelectronic devices.
Porphyrin and phthalocyanine are structurally related macrocyclic compounds, containing four pyrrole-like subunits linked to form 16-membered ring. Owing to their highly conjugated π- electron systems, both porphyrin and phthalocyanine show intense absorptions in the visible region and exceptional thermal and chemical stability [24, 25]. More importantly, their photophysical and redox properties can be tuned by carefully choosing the metal center or peripheral substituents [26-28]. As such, they have been serving as important components of molecular materials that possess unique electronic [29-31], magnetic [32] and physicochemical properties [33-35]. The incorporation of porphyrin or phthalocyanine into 2D COFs, which should allow these π-electron systems align in a precise order and open the possibilities of resulting porous materials as semiconductors or emission solids, has also been investigated in the past five years. Various 2D porphyrin- or phthalocyanine-based COFs, denoted as Por-COFs or Pc-COFs, have been reported and these systems have shown interesting applications from catalysis to molecular electronic devices.
In this review, we will summarize the research progress of 2D Por-COFs and Pc-COFs. We start with the synthesis of these 2D COFs by using various dynamic covalent reactions. After that, we will emphasize their potential applications in different areas. At the end, perspectives are also mentioned, regarding to their remaining challenging issues.
2. Synthesis of 2D Por-COFs and Pc-COFsDynamic covalent chemistry [36, 37], which can allow the formation of covalent bonds with “error checking” and “proofreading”, has been utilized to construct COFs via solvothermal method under thermodynamic control [38]. Considering the geometry of porphyrin or phthalocyanine and the synthetic challenge, only a few functional porphyrin or phthalocyanine precursors (Fig. 1) were designed and synthesized, which further reacted with different linkers (Fig. 1) to construct Por-COFs or Pc- COFs. In this section, we will briefly introduce the synthesis of 2D Por-COFs and Pc-COFs by using several dynamic covalent reactions.

|
Download:
|
| Figure 1. Building blocks that have been utilized for the synthesis of two-dimensional porphyrin- and phthalocyanine-based covalent organic frameworks. | |
2.1. Synthesis of 2D Por-COFs
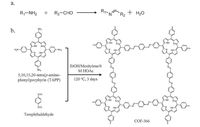
The reversible condensation between aldehydes and amines is one of the most ubiquitous and oldest reactions in organic chemistry (Fig. 2a). By utilizing this reaction, several 2D Por-COFs were synthesized. In 2011, Yaghi and co-workers reported [39] the synthesis and characterization of the first imine-based Por-COF (COF-366, Fig. 2b), starting from 5, 10, 15, 20-tetra(π-amino-phe- nyl)porphyrin (TAPP, 1) and terephthaldehyde (16). Later on, by using metallized TAPP as the precursors, Jiang and co-workers reported [40] a series of metalloporphyrin-based COFs, which have shown that the focal metals in the porphyrin ring play a dominating role on the properties of resulting COFs. Very recently, we reported [41] a 2D Por-COF synthesized from 5, 10, 15, 20- tetrakis (4-benzaldhyde)porphyrin (5) and p-phenylenediamine (27), which has a higher Brunauer-Emmett-Teller (BET) surface area than that of its analog, COF-366.
|
Download:
|
| Figure 2. Schematic representation of (a) imine bonds formation; (b) synthesis of COF-366. | |
|
Download:
|
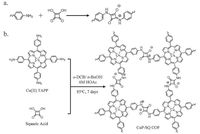
| Figure 3. Schematic representation of (a) squaraine formation reaction; (b) synthesis of CuP-SQ COF. | |
The condensation of boronic acids with diols to give boronate esters (Fig. 4a) has been proven to be a highly efficient strategy for preparing complicated structures and this reaction has also been utilized to synthesize 2D Por-COFs. In 2011, Yaghi and co-workers reported [39] the first boronate ester-linked Por-COF (COF-66), formed by condensation of tetra(π-boronic acid-phenyl)porphyrin (TBPP, 6) and 2, 3, 4, 5-tetrahydroxy anthracene (25). After a while, Jiang and co-workers reported [42] several boronate ester-linked Por-COFs, condensed by TBPP or metal (Zn and Cu) TBPP with 1, 2, 4, 5-tetrahydroxybenzene (24). Recently, Bein and co-workers reported [43] a new boronate ester-linked Por-COF (Tp-Por COF, Fig. 3a), starting from 5, 15-bis(4-boronophenyl)-porphyrin (BBPP, 9) and 2, 3, 6, 7, 10, 11-hexahydroxytriphenylene (26).

|
Download:
|
| Figure 4. Schematic representation of (a) boronate ester formation; (b) synthesis of Pc-PBBA COF. | |
In addition to these two strategies, Jiang and co-workers developed [44] a new method to synthesize Por-COF based on squaraine chemistry (Fig. 3a). Starting from copper (II) TAPP (4) and squaric acid (28), they successfully obtained a squaraine- linked Por-COF (CuP-SQ COF, Fig. 3b), which exhibits enhanced chemical and thermal stabilities and features a very broad range of light absorption.
2.2. Synthesis of 2D Pc-COFsUnlike 2D Por-COFs, all the reported 2D Pc-COFs are constructed through boronate ester linkage. In 2010, Dichtel and coworkers reported [45] the first 2D Pc-COF synthesized from the condensation of phthalocyanine tetra(acetonide) (10) and 1, 4- phenylenebis(boronic acid) (29) under the catalysis of Lewis acid BF3 OEt2 (Fig. 4b). Later on, they reported [46] several metal- lophthalocyanine-based COFs, by condensation of Zn octahydrox- yphthalocyanine (15) and different linkers (31, 32, 33, 34). By increasing the length of linkers, Pc-COFs with pore size up to 4.4 A were obtained. Jiang and co-workers also reported several boronate ester-linked Pc-COFs. For example, starting from (2, 3, 9, 10, 16, 17, 23, 24-octahydroxyphthalocyaninato)nickel (13) and 1, 4-benzothiadiazole diboronic acid (35), an n-type 2D Pc- COF (NiPc-BTDA COF) with enhanced absorbance over a wide range of wavelengths up to 1000 nm was synthesized [47]. We should mention here, the boronate ester-linked Pc-COFs and Por-COFs can be hydrolyzed in moist atmosphere or water, which may restrict their further application.
3. Functionalization of 2D Por-COFs and Pc-COFsThe modification and functionalization of COFs should benefit the resulting materials with interesting properties. As such, the functionalization of 2D Por-COFs or Pc-COFs has also been investigated, mainly by using post-synthetic modification (PSM) approach. Click chemistry, which can proceed the reaction efficiently with high yields and high specificity in the presence of various functional groups [48], has been applied to functionalize 2D Por-COFs and Pc-COFs. For example, Jiang and co-workers reported a series of Por-COFs with different loading of alkyne units (22), which can integrate organocatalytic sites into the pore walls for targeting organocatalytic COFs through click reactions [49]. They also reported a series of metallized Pc-COFs with different amounts of azide units (38) that can react with alkyne-functionalized fullerene targeting aligned donor-acceptor COFs [50].
In addition, Jiang and co-workers reported the utilizing of another reaction to modify the pore wall of Por-COFs. They synthesized a series of Por-COFs with different contents of phenol groups, which can undergo a quantitative ring opening reaction with succinic anhydride, toward decorating the channel wall with open carboxylic acid groups [51].
4. Applications of 2D Por-COFs and Pc-COFsAs predesignable porous materials, 2D Por-COFs and Pc-COFs have shown interesting applications in many areas. In this section, we will summarize the applications progress of 2D Por-COFs and Pc-COFs.
4.1. Gas storageThe porous nature of Por-COFs and Pc-COFs endows these materials with application for gas storage. In 2013, Echegoyen and co-workers reported [52] a phthalocyanine and porphyrin 2D COF constructed from octahydroxy phthalocyanine Co(II) (12) and BBPP (9), which shows a hydrogen storage capacity up to 0.8 wt% at 77 K and 1 bar. Later on, they reported [53] another Pc-COF condensed from 12 and 4, 4'-biphenyl bisboronic acid (30), which has high surface area (SBET = 1087 m2 g-1) and can store up to 1.2 wt% of hydrogen at 77 K and 1 bar. Moreover, from density functional theory (DFT) calculations, the Li doped 2D Por-COFs or Pc-COFs may have high hydrogen storage capacity [54, 55].
The CO2 storage behavior of 2D Por-COFs has also been studied. For example, Echegoyen and co-workers reported [56] a Por-COF that can store CO2 up to 5.5 wt% at 273 K and 1 bar, even the crystallinity is poor. In addition, the carboxylic acid decorated Por- COF, synthesized via a PSM approach [51], shows outstanding carbon dioxide capture ability up to 18.0 wt% at 273 K and 1 bar.
4.2. CatalysisSince boronate ester-linked COFs are unstable in a humid environment, the application of Pc-COFs in catalysis has not been reported yet. Herein, we mainly focus on the catalysis behavior of Por-COFs.
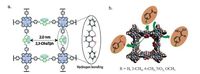
If designed properly, 2D Por-COFs can be used as organocatalyst. For example, as shown in Fig. 5, Banerjee and co-workers reported [57] a 2D Por-COF (2, 3-DhaTph) synthesized from TAPP and 2, 3-dihydroxyterephthalaldehyde (17), in which the catecholic-OH groups on the linker act as weak acidic sites while the porphyrin units and imine bonds act as basic sites. This particular COF can catalyze cascade reaction, with good selectivity and reusability. In addition, Jiang and co-workers reported the construction of covalently linked and highly active organocatalytic Por-COF through PSM approach, which exhibits enhanced activity in asymmetric Michael addition reactions with retaining stereoselectivity [49].
|
Download:
|
| Figure 5. (a) Structure of 2, 3-DhaTph and its intrinsic hydrogen bonding to enhance the structure stability. (b) Schematic representation of the cascade reaction catalyzed by 2, 3- DhaTph. Source: Reprinted with permission from Ref. [57]. Copyright (2015) The Royal Society of Chemistry. | |
|
Download:
|
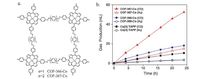
| Figure 6. (a) Structures of COF-366-Co and COF-367-Co. (b) Long-term bulk electrolysis of CO2 solution at -0.67 V (versus RHE) by COF-367-Co (red triangles), COF-366-Co (blue circles), and Co(II) TAPP (the precursor, black squares) with producing carbon monoxide (solid dots) and H2 (open dots). Source: Reprinted with permission from Ref. [58]. Copyright (2015) AAAS. | |
Besides, the incorporation of metallophorphyrins into COFs can possess the resulting COFs with interesting properties, such as electrocatalysis. In 2015, Yaghi and co-workers reported [58] a series of 2D Co(II) Por-COFs (Fig. 6), which can efficiently reduce CO2 to CO in water. For example, COF-367-Co constructed from Co(II) TAPP and biphenyl- 4, 4'-dicarboxaldehyde (23) can catalyze this reduction at -0.67 V with high Faradaic efficiency (91%) and turnover number (3901), with a turnover frequency (TOF) of 165 h-1. In addition, they prepared bimetallic (Co and Cu) COF-367 derivatives, which show a substantial improvement in electrocatalysis with each 10-fold dilution of cobalt loading. Moreover, they directly synthesized COF onto the surface of an electrode substrate in the form of oriented thin films. Under the same electrolysis condition, the thin films on glassy carbon exhibited a TOF of 665 h-1, a value seven times as high as that of the same material deposited on a carbon fabric.
Except for organo- and electro-catalysis, some 2D Por-COFs also show photo-catalysis behavior. For example, the CuP-SQ COF mentioned in Section 2.1 can harvest a wide range of photons from the visible to deeπ-red regions, thus showing excellent catalysis ability for the activation of molecular oxygen into singlet oxygen [44].
4.3. Semiconduction and photoconduction2D Por-COFs and Pc-COFs provide a new platform for facilitating the transport of charge carriers and photoexcited
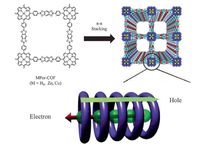
|
Download:
|
| Figure 7. Structure ofMPor-COFs and schematic representation of their stacking to form π-columns for charge transport. (Purple circles: porphyrin rings for hole conduction; Green balls: focal metal ions for electron conduction.) Source:Reprinted with permission from Ref. [42]. Copyright (2015) Wiley. | |

|
Download:
|
| Figure 8. (a) Structure ofTP-Por-COF. (b) The DPV measured frontier orbital energies of subunits in solution and schematically represented photoinduced charge transfer. (c) Cross-sectional SEM image of the sandwiched photovoltaic device (The MoOx and ZnO contact layers are too thin to be visible). (d) The current-voltage curve of the photovoltaic device. Source: Reprinted with permission from Ref. [43]. Copyright (2015) American Chemical Society. | |
states through pre-organized pathways. By using laser flash- photolysis time-resolved microwave conductivity (FP-TRMC) methods, the intrinsic charge carrier mobility of 2D Por-COFs and Pc-COFs has been investigated. For example, COF-366 and COF-66 were determined to be hole conducting with the highest charge carrier motility value (8.1 and 3.0 cm2 V-1 s-1) among known organic crystalline conducting polymers [39]. Boronate ester-linked Por-COFs (MPor-COFs, Fig. 7), depending on the central metals in porphyrin macrocycle, can switch the conducting nature from hole to electron as well as to ambipolar [42]. Similar to Por-COFs, Pc-COFs also exhibited high carrier mobility due to the formation of periodic phthalocyanine π-columns [59].
The eclipsed stacking structure and alignment of the π- conjugated units endow 2D Por-COFs and Pc-COFs with a probability of triggering photoconductivity [60]. For example, some 2D Por-COFs and Pc-COFs exhibit a significant increase in current upon irradiation with a high on-off ratio [42, 47]. If the intrinsic photocurrent is able to be extracted out, then it is possible to use 2D Por-COFs and Pc-COFs for developing high-performance photovoltaic devices. Recently, Bein and co-workers reported the first example of a photovoltaic device that utilizes Tπ-Por-COF as the active layer (Fig. 8), which was found to promote charge separation upon photoexcitation [43]. After the construction of a device by further sandwiching Tπ-Por-COF between indium tin oxide (ITO)/MoOx and ZnO/Al electrodes, an open-circuit voltage of 312 mV and a short circuit current density of 44.6 mAcm-2 were detected under illuminating with simulated solar light. Moreover, the external quantum efficiency under reverse bias shows this kind of devices may have a chance to replace the currently used small molecule/fullerene-containing devices.
5. Conclusion and perspectiveAs an important class of porous crystalline polymers with predictable structures and tunable pore functionality, COFs have gained considerable attention over the past decade. 2D Por-COFs and Pc-COFs, which allow the π-conjugated porphyrin or phthalocyanine align in a precise order, have been relatively well-explored in the past five years. By covering most of the reported work, this review summarized the research progress in the synthesis, functionalization and application of 2D Por-COFs and Pc-COFs. We should mention here, although a lot of progress had been made, there are still some challenges need to be further addressed. For example, only a few reactions have been utilized to construct 2D Por-COFs and Pc-COFs until now, thus more synthetic tools are earnestly expected. In addition, for a prolonged usage, their stability also needs to be further concerned and improved. Furthermore, as the thin-film of 2D Por-COFs and Pc-COFs may show remarkable performance in optoelectronic devices, film growth of these COFs with high quality are highly demanded. Therefore, an ever increasing expansion in this attractive area can be imagined in the future.
Acknowledgments This work was supported by National Natural Science Foundation of China (No. 21572170), the Research Fund for the Doctoral Program of Higher Education of China (No. 20130141110008), the Outstanding Youth Foundation of Hubei Province (No. 2015CFA045), the Beijing National Laboratory for Molecular Sciences and the Open Foundation of State Key Laboratory of Electronic Thin Films and Integrated Devices (No. KFJJ201505).| [1] | S.Y. Ding, W. Wang. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42 (2013) 548–568. DOI:10.1039/C2CS35072F |
| [2] | X.M. Liu, J. Guo, X. Feng, J.H. Dong, Covalent organic frameworks materials and two-dimensional macromolecules, Bull Natl. Nat. Sci. Found. China (2014) 330-339. |
| [3] | W. Wang. Porous organic polymers: a new star in porous materials. Acta Chim. Sinica 73 (2015) 461–462. DOI:10.6023/A1506E001 |
| [4] | S.S. Han, H. Furukawa, O.M. Yaghi, W.A. Goddard III. Covalent organic frameworks as exceptional hydrogen storage materials. J. Am. Chem. Soc. 130 (2008) 11580–11581. DOI:10.1021/ja803247y |
| [5] | T.Y. Zhou, S.Q. Xu, Q. Wen, Z.F. Pang, X. Zhao. One-step construction of two different kinds of pores in a 2D covalent organic framework. J. Am. Chem. Soc. 136 (2014) 15885–15888. DOI:10.1021/ja5092936 |
| [6] | Z.P. Li, Y.F. Zhi, X. Feng, et al. An azine-linked covalent organic framework: synthesis, characterization and efficient gas Storage. Chem. Eur. J. 21 (2015) 12079–12084. DOI:10.1002/chem.v21.34 |
| [7] | Q. Gao, L.Y. Bai, X.J. Zhang, et al. Synthesis of microporous nitrogen-rich covalentorganic framework and its application in CO2 capture. Chin. J. Chem. 33 (2015) 90–94. DOI:10.1002/cjoc.v33.1 |
| [8] | Y.F. Zeng, R.Q. Zou, Y.L. Zhao. Covalent organic frameworks for CO2 capture. Adv. Mater. 28 (2016) 2855–2873. DOI:10.1002/adma.201505004 |
| [9] | Z.X. Kang, Y.W. Peng, Y.H. Qian, et al. Mixed matrix membranes (MMMs) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 28 (2016) 1277–1285. DOI:10.1021/acs.chemmater.5b02902 |
| [10] | S.Y. Ding, J. Gao, Q. Wang, et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 133 (2011) 19816–19822. DOI:10.1021/ja206846p |
| [11] | Q.R. Fang, S. Gu, J. Zheng, et al. 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis,. Angew. Chem. Int. Ed 53 (2014) 2878–2882. DOI:10.1002/anie.v53.11 |
| [12] | Y.F. Xie, S.Y. Ding, J.M. Liu, W. Wang, Q.Y. Zheng. Triazatruxene based covalent organic framework and its quick-response fluorescence-on nature towards electron rich arenes. J. Mater. Chem. C 3 (2015) 10066–10069. DOI:10.1039/C5TC02256H |
| [13] | G.Q. Lin, H.M. Ding, D.Q. Yuan, B.S. Wang, C. Wang. A pyrene-based, fluorescent three-dimensional covalent organic framework. J. Am. Chem. Soc. 138 (2016) 3302–3305. DOI:10.1021/jacs.6b00652 |
| [14] | M. Dogru, T. Bein. On the road towards electroactive covalent organic frameworks. Chem. Commun. 50 (2014) 5531–5546. DOI:10.1039/C3CC46767H |
| [15] | J. Guo, Y.H. Xu, S.B. Jin, et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4 (2013) 2736. |
| [16] | C.R. DeBlase, K.E. Silberstein, T.T. Truong, H. D.. Abruña, W.R. Dichtel, β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 135 (2013) 16821–16824. DOI:10.1021/ja409421d |
| [17] | H.P. Liao, H.M. Ding, B.J. Li, X.P. Ai, C. Wang. Covalent-organic frameworks: potential host materials for sulfur impregnation in lithium-sulfur batteries. J. Mater. Chem. A 2 (2014) 8854–8858. DOI:10.1039/c4ta00523f |
| [18] | C.R. DeBlase, K. Hernández-Burgos, K.E. Silberstein, et al. Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 9 (2015) 3178–3183. DOI:10.1021/acsnano.5b00184 |
| [19] | B. Lukose, A. Kuc, T. Heine. The structure of layered covalent-organic frameworks. Chem. Eur. J. 17 (2011) 2388–2392. DOI:10.1002/chem.201001290 |
| [20] | P. Zhu, V. Meunier. Electronic properties of two-dimensional covalent organic frameworks. J. Chem. Phys. 137 (2012) 244703. DOI:10.1063/1.4772535 |
| [21] | G.H.V. Bertrand, V.K. Michaelis, T.C. Ong, R.G. Griffin, M. Dincă. Thiophene-based covalent organic frameworks. Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 4923–4928. DOI:10.1073/pnas.1221824110 |
| [22] | H.M. Ding, Y.H. Li, H. Hu, et al. A tetrathiafulvalene-based electroactive covalent organic framework. Chem. Eur. J. 20 (2014) 14614–14618. DOI:10.1002/chem.v20.45 |
| [23] | W.L. Dong, L. Wang, H.M. Ding, et al. Substrate orientation effect in the on-surface synthesis of tetrathiafulvalene-integrated single-layer covalent organic frameworks. Langmuir 31 (2015) 11755–11759. DOI:10.1021/acs.langmuir.5b02412 |
| [24] | R. Bonnett. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 24 (1995) 19–33. DOI:10.1039/cs9952400019 |
| [25] | A. Yella, H.W. Lee, H.N. Tsao, et al. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334 (2011) 629–634. DOI:10.1126/science.1209688 |
| [26] | J.R. Darwent, P. Douglas, A. Harriman, G. Porter, M.C. Richoux. Metal phthalocyanines and porphyrins as photosensitizers for reduction of water to hydrogen. Coord. Chem. Rev. 44 (1982) 83–126. DOI:10.1016/S0010-8545(00)80518-4 |
| [27] | P.G. Gassman, A. Ghosh, J. Almlof. Electronic effects of peripheral substituents in porphyrins: X-ray photoelectron spectroscopy and ab initio self-consistent field calculations. J. Am. Chem. Soc. 114 (1992) 9990–10000. DOI:10.1021/ja00051a035 |
| [28] | P. Kar, S. Sardar, E. Alarousu, et al. Impact of metal ions in porphyrin-based applied materials for visible-light photocatalysis: key information from ultrafast electronic spectroscopy. Chem. Eur. J. 20 (2014) 10475–10483. DOI:10.1002/chem.v20.33 |
| [29] | X.X. Ma, Q.Z. Ren, Z.F. Ma. Progress of studies on metalloporphyrin compounds as electrocatalysts. Chem. World 46 (2005) 243–246. |
| [30] | M.U. Winters, E. Dahlstedt, H.E. Blades, et al. Probing the efficiency of electron transfer through porphyrin-based molecular wires. J. Am. Chem. Soc. 129 (2007) 4291–4297. DOI:10.1021/ja067447d |
| [31] | C.G. Claessens, U. Hahn, T. Torres. Phthalocyanines: from outstanding electronic properties to emerging applications. Chem. Rec. 8 (2008) 75–97. DOI:10.1002/(ISSN)1528-0691 |
| [32] | W. Liu, H.C. Zhang, J.Z. Jiang. Phthalocyanine & porphyrin-based molecular magnets: synthesis, structure characteristics and applications, J. Chin. Rare Earth Soc. 20 (2001) 1–10. |
| [33] | F. D'Souza, G.R. Deviprasad, M.E. El-Khouly, M. Fujitsuka, O. Ito. Probing the donor-acceptor proximity on the physicochemical properties of porphyrin-fullerene dyads: "Tail-On" and "Tail-Off" binding approach. J. Am. Chem. Soc. 123 (2001) 5277–5284. DOI:10.1021/ja010356q |
| [34] | M.O. Senge, M. Fazekas, E.G.A. Notaras, et al. Nonlinear optical properties of porphyrins. Adv. Mater. 19 (2007) 2737–2774. DOI:10.1002/(ISSN)1521-4095 |
| [35] | J.B. Zhang, P.Y. Zhang, G.H. Chen, F. Han, X.H. Wei. Photochemical reaction between magnesium tetraphenyl porphyrin and oxygen. Chin. Chem. Lett. 19 (2008) 1190–1192. DOI:10.1016/j.cclet.2008.07.004 |
| [36] | S.J. Rowan, S.J. Cantrill, G.R.L. Cousins, J.K. Sanders, J.F. Stoddart. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41 (2002) 898–952. DOI:10.1002/1521-3773(20020315)41:6<>1.0.CO;2-R |
| [37] | Y.H. Jin, C. Yu, R.J. Denman, W. Zhang. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 42 (2013) 6634–6654. DOI:10.1039/c3cs60044k |
| [38] | B.L. Zhou, L. Chen. New strategies for the synthesis of covalent organic porous polymers. Acta Chim. Sinica 73 (2015) 487–497. DOI:10.6023/A15020090 |
| [39] | S. Wan, F. Gándara, A. Asano, et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23 (2011) 4094–4097. DOI:10.1021/cm201140r |
| [40] | X. Chen, M. Addicoat, E.Q. Jin, et al. Locking covalent organic frameworks with hydrogen bonds: general and remarkable effects on crystalline structure, physical properties, and photochemical activity. J. Am. Chem. Soc. 137 (2015) 3241–3247. DOI:10.1021/ja509602c |
| [41] | H.P. Liao, H.M. Wang, H.M. Ding, et al. A 2D porous porphyrin-based covalent organic framework for sulfur storage in lithium-sulfur batteries. J. Mater. Chem. A 4 (2016) 7416–7421. DOI:10.1039/C6TA00483K |
| [42] | X. Feng, L.L. Liu, Y. Honsho, et al. High-rate charge-carrier transport in porphyrin covalent organic frameworks: switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 51 (2012) 2618–2622. DOI:10.1002/anie.201106203 |
| [43] | M. Calik, F. Auras, L.M. Salonen, et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 136 (2014) 17802–17807. DOI:10.1021/ja509551m |
| [44] | A. Nagai, X. Chen, X. Feng, et al. A squaraine-linked mesoporous covalent organic framework. Angew. Chem. Int. Ed. 52 (2013) 3770–3774. DOI:10.1002/anie.201300256 |
| [45] | E.L. Spitler, W.R. Dichtel. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2 (2010) 672–677. DOI:10.1038/nchem.695 |
| [46] | E.L. Spitler, J.W. Colson, F.J. Uribe-Romo, et al. Lattice expansion ofhighly oriented 2D phthalocyanine covalent organic framework films. Angew. Chem. Int. Ed. 51 (2012) 2623–2627. DOI:10.1002/anie.201107070 |
| [47] | X.S. Ding, L. Chen, Y. Honsho, et al. An n-channel two-dimensional covalent organic framework. J. Am. Chem. Soc. 133 (2011) 14510–14513. DOI:10.1021/ja2052396 |
| [48] | H.C. Kolb, M.G. Finn, K.B. Sharpless. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40 (2001) 2004–2021. DOI:10.1002/(ISSN)1521-3773 |
| [49] | H. Xu, X. Chen, J. Gao, et al. Catalytic covalent organic frameworks via pore surface engineering. Chem. Commun. 50 (2014) 1292–1294. DOI:10.1039/C3CC48813F |
| [50] | L. Chen, K. Furukawa, J. Gao, et al. Photoelectric covalent organic frameworks: converting open lattices into ordered donor-acceptor heterojunctions. J. Am. Chem. Soc. 136 (2014) 9806–9809. DOI:10.1021/ja502692w |
| [51] | N. Huang, X. Chen, R. Krishna, D.L. Jiang. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. Int. Ed. 54 (2015) 2986–2990. DOI:10.1002/anie.201411262 |
| [52] | V.S.P.K. Neti, X.F. Wu, S.G. Deng, L. Echegoyen. Synthesis of a phthalocyanine and porphyrin 2D covalent organic framework. CrystEngComm 15 (2013) 6892–6895. DOI:10.1039/c3ce40706c |
| [53] | V.S.P.K. Neti, X.F. Wu, M. Hosseini, et al. Synthesis of a phthalocyanine 2D covalent organic framework. CrystEngComm 15 (2013) 7157–7160. DOI:10.1039/c3ce41091a |
| [54] | J.H. Guo, H. Zhang, Z.P. Liu, X.L. Cheng. Multiscale study of hydrogen adsorption, diffusion, and desorption on Li-doped phthalocyanine covalent organic frameworks. J. Phys. Chem. C 116 (2012) 15908–15917. DOI:10.1021/jp305949q |
| [55] | P. Srepusharawoot, E. Swatsitang, V. Amornkitbamrung, U. Pinsookd, R. Ahujag. Hydrogen adsorption of Li functionalized Covalent Organic Framework-366: an ab initio study. Int. J. Hydrogen Energy 38 (2013) 14276–14280. DOI:10.1016/j.ijhydene.2013.08.102 |
| [56] | V.S.P.K. Neti, X.F. Wu, S.G. Deng, L. Echegoyen. Selective CO2 capture in an imine linked porphyrin porous polymer. Polym. Chem. 4 (2013) 4566–4569. DOI:10.1039/c3py00798g |
| [57] | D.B. Shinde, S. Kandambeth, P. Pachfule, R.R. Kumar, R. Banerjee. Bifunctional covalent organic frameworks with two dimensional organocatalytic micropores. Chem. Commun. 51 (2015) 310–313. DOI:10.1039/C4CC07104B |
| [58] | S. Lin, C.S. Diercks, Y.B. Zhang, et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349 (2015) 1208–1213. DOI:10.1126/science.aac8343 |
| [59] | X.S. Ding, X. Feng, A. Saeki, et al. Conducting metallophthalocyanine 2D covalent organic frameworks: the role of central metals in controlling p-electronic functions. Chem. Commun. 48 (2012) 8952–8954. DOI:10.1039/c2cc33929c |
| [60] | S.B. Jin, M. Supur, M. Addicoat, et al. Creation ofsuperheterojunction polymers via direct polycondensation: segregated and bicontinuous donor-acceptor π-columnar arrays in covalent organic frameworks for long-lived charge separation. J. Am. Chem. Soc. 137 (2015) 7817–7827. DOI:10.1021/jacs.5b03553 |
 2016, Vol. 27
2016, Vol. 27 


