Materials that are stimuli-responsive have raised tremendous attentions because of their potential technological applications as smart photoactive systems. The stimuli response for emitting colour changes includes many mechanisms, such as mechan- ochromism [1], vapochromism [2], thermochromism [3], acid- dependent luminescence [4], and photochromism [5]. Mechan- ochromism is the mechanisms of mechanochromic phenomenon that induced by mechanical stimuli. Both visible and luminescence colours (emission wavelength and intensity) are responsive to the external force. Materials scientists show growing interests on mechanochromic organic materials in the past decades due to their potential applications in sensors and memory devices. These materials are mostly investigated and largely documented in literatures due to the fact that a convenient operation, ground by a spoon or mortar, can effectively perturb the molecular conformation and/or arrangements and hence lead to chromism. In sharp contrast, there are only a few reports that describe mechan- ochromic actualized by isotropic compression because strict operating conditions are required for high-pressure measurement.
Generally, the luminescent properties of organic materials are determined by chemical structures and the desired properties can be realized by suitable molecular design. However, in the aggregated state, molecular configuration and supramolecular interactions will greatly affect the luminescence of an organic compound [6-10]. Under high pressure, molecular geometry (distances and angles) and energy (vibrational and optical absorption band wavenumbers, intensities, widths) of organic materials are usually different to that in ambient condition; this will lead to variations of properties, particularly for mechan- ochromic materials. Organic crystals can undergo deformation accompanied by the generation of mechanoluminescence when external forces are applied. Compressing, grinding, and smashing are three major operations which produced isotropic pressure, anisotropic pressure and tensile force, respectively, on crystalline samples. Among which, isotropic compression have unique effects on crystals because the very high isotropic hydrostatic pressure can significantly modify both molecular conformation and packing of crystals without the destruction of crystal shape.
To achieve high pressure conveniently and effectively, the Diamond Anvil Cell (DAC) equipment is a simply way. So far there are only a few reports concentrate on the study of mechan- ochromic phenomenon of organic materials under high pressure due to that DAC is not a common technic. This mini-review present a general overview on some recent achievements in studying mechanochromic organic materials combined with DAC.
2. The basic concept of DACHydrostatic pressure has been proven to be effective in tuning properties of organic and inorganic materials. High pressures even over hundreds of GPa are found to be quite difficult but possible to achieve based on the equipment of DAC which was developed in the 1986 (550 Gpa) and in the 2012 (600 Gpa) [11, 12]. The interatomic spacing as well as intermolecular distance will be greatly shortened when high pressure is applied. In addition, weak intermolecular interactions such as hydrogen bonding and π-π interactions are sensitive to the applied pressures (Scheme 1).
|
Download:
|
| Scheme. 1. Chemical structure of compounds 1-13. | |
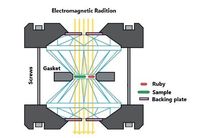
The in situ characterizations based on DAC, including X-ray diffraction (XRD), fluorescence spectroscopy, Raman scattering, ultraviolet-visible (UV-vis) absorption, and infrared (IR), provide detailed insight into the pressure-induced structural changes. Thanks to the technological advances of DAC methods, high pressure research has been explored extensively, particularly in material science. The basic working principle of the DAC is illustrated in Fig. 1. A metal gasket hole is located between the flat and parallel surface of two diamond anvils in which samples are loaded. When tighten the screws, the backing plate forced two opposed anvils together. As a result, the sample in the metal gasket hole was subjected to high pressure. Liquid pressure transmitting medium was employed to fill in the pressure chamber which
|
Download:
|
| Figure 1. Schematic of diamond anvil cell. | |
generate the hydrostatic pressure conditions. In addition, a well- calibrated ruby was used to determine the pressure values inside the DAC sample chamber. Raman scattering, X-ray diffraction (XRD), infrared (IR), ultraviolet-visible (UV-vis) absorption, fluorescence spectroscopy and other measurements that performed as in situ characterizations of DAC, could offer the opportunities to study the pressure-induced changes of intermolecular interactions as well as molecular structures and thus provide insights into the mechanism of mechanochoromic behaviors of organic materials [13, 14].
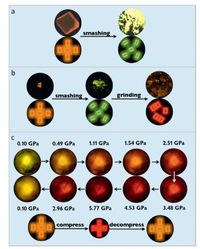
3. Mechanochromic luminescent materials 3.1. Organoboron compoundsFour-coordinate organoboron compounds with rigid π-conju- gated structures are intensely luminescent and have high carrier mobility which enables them to be applied in optoelectronics including organic light-emitting diodes (OLEDs), and organic solid- state lasers (OSLs) [15-17]. Recently, Zhang et al designed a novel boron-containing material 1 which exhibited mechanochromic properties. This indicates the application of four-coordinate boron- containing materials can be expanded to multifunctional sensors or memory devices [18]. Polymorph 1OC of this boron material adopted a metastable molecular packing which can be readily perturbed by external stresses like tensile forces, anisotropic and isotropic pressures through mechanical smashing, grinding and compressing, respectively (Fig. 2).
|
Download:
|
| Figure 2. Photographic images and molecular arrangement transformation of (a) 1OC crystal before and after smashing, (b) fragmental crystals before and after grinding and (c) 1OC crystal under different isotropic pressures. Adapted from Ref. [18] with permission from The John Wiley and Sons. | |
Upon smashing of 1OC by a stainless steel spoon, the fluorescence intensity of the sample was significantly enhanced accompanied with the emission band blueshifted from 585 nm to 565 nm. This result showed that the tensile force disrupted the weak intermolecular interaction between dimers, thus lead to blueshifted emission and enhanced luminescent intensity. Further ground the fragmental crystals with a mortar, a weakly red luminescent powder with a broad emission band centered at 605 nm was obtained. The fragmental crystals and original 1OC crystals were converted into an amorphous powder, which showed redshifted luminescence, due to the different molecular arrangements such as π-stacked dimers induced by grinding. 1OC crystals presented a significant reddshifted emission from 585 nm to 760 nm under isotropic pressure. The emission change could be reversed upon decompression, thus indicating that the packing mode of the molecules did not undergo a substantial change during the high-pressure experiment. Compression may shorten the distance between the p planes in a dimer composed of two molecules, and hence lead to redshifted luminescence and reduced emission intensity.
The same research group employed another four-coordinate boron-containing material 2 and observed the similar high- pressure induced emission behavior [19]. The molecular structure of compound 2 is very similar to compound 1. With an in-depth study of luminescent properties, they found an intriguing phenomenon of realizing RGB primary emissions (R: red, G: green, B: blue) based on the single organic compound 2. The solid-state luminescence of this material was found to be responsive to multiple external stimuli like pressure, heat, and acid. Two polymorphs 2OC and 2RC were obtained by either solvent diffusion or vacuum sublimation approaches. The orange crystals 2OC and red crystals 2RC showed fluorescent spectrum with emission peak located at about 580 nm and 615 nm, respectively. The luminescence of 2OC displayed distinct response towards mechanical grinding and hydrostatic pressures. 2OC readily converted into orange red solids 2OR after simple mechanical grinding accompanied by the emission peak shifted from 580 nm to 610 nm. Upon applying certain hydrostatic pressure produced by DAC, the luminescence of 2OC solids significantly redshifted to 655 nm (Fig. 3). The high-pressure induced mechanochromic properties of the boron-containing materials provide an ideal model to reveal the relationship between intermolecular distance and luminescent property of organic solids. In these four- coordinate boron-containing organic crystals, high pressures produced by DAC mainly affect the intermolecular π-πlane distance which decided the emission properties of the bulk crystals.

|
Download:
|
| Figure 3. (a) The luminescent spectra and (b) micrographs of the crystal 2OC under different pressures.Adapted from Ref. [19] with permission from The Royal Society of Chemistry. | |
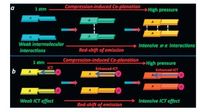
Recently, Liu and coworkers report two new propeller-shaped benzothiazole-enamido boron difluoride complexes 3 and 4 which exhibited mechanochromic luminescence upon hydrostatic compressing [20]. Both compounds are weakly fluorescent in organic solvents with low viscosity, but brightly emissive in high-viscosity solvent such as glycerol. Compound 3 did not show obvious luminescence shift when the solvent was change from hexane to glycol, while a distinct bathochromic shift from 478 nm (in hexane) to 522 nm (in glycol) was observed for compound 4, indicating that compound 4 has an intramolecular charge transfer (ICT) character. Both boron complexes 3 and 4 displayed obvious redshift in fluorescent spectra after applying high pressure. For 3 the luminescence maximum of the band was shifted from 473 nm to 571 nm at 10.84 GPa, and for 4 the emission peak changed from 518 to 645 nm at the same pressure condition. Compound 4 showed more sensitive piezochromic response at low pressure (< 1.5 GPa) than compound 3. Compound 3 showed the dual band luminescence and the distinct hysteresis loop during the compression-decompression process, while compound 4 always showed a single band luminescence. The authors proposed mechanism of the different piezochromic behaviors of 3 and 4 as follows: the compression-induced luminophore co-planation led to the distinct enhancement of π-π intermolecular interaction in compound 3 but the intramolecular CT effect in compound 4, which were proposed to be responsible for their different piezochromic luminescence (Fig. 4). The enhanced π-π intermolecular interaction favored the excimer formation, resulting in the dual band luminescence (excimer and monomer luminescence) of compound 3. This report proved that the pressure-dependent π-π intermolecular interaction and the intramolecular CT effect were efficient in inducing mechanochromic luminescence. The present example indicates that the DAC technique can finely tune the mechanoluminescence through different mechanism even in a very similar molecular system.
|
Download:
|
| Figure 4. Proposed mechanisms of the different piezochromic behaviors for compounds 3 (a) and 4 (b).Adapted from Ref. [20] with permission from The Royal Society of Chemistry. | |
According to the results summarized above, four-coordinate boron-containing compounds are good candidates for constructing organic crystals with pressure-induced mechanoluminescence. Notably, the piezochromic behaviors of organoboron crystals under high-pressure conditions can be controlled through properly structural design.
3.2. CN-DSB derivativesDistyrylbenzene (DSB) was a well-known π-conjugated framework with planar configuration and strong blue fluorescence and has been widely employed as structural unit in constructing organic functional materials [21]. When the vinyl in DSB was substituted by cyano, the resultant derivative termed CNDSB have multiform molecular configurations and crystalline structures which lead to various interesting optical properties. There have been many reports on the crystal structures of CN-DSB and its derivatives [22-24].
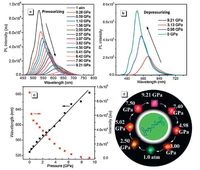
Very recently, Lu and coworkers synthesized an organic molecule 1, 4-bis(1-cyano-2-phenylethenyl)benzene (CNDSB) 5 and reported the unique piezochromic fluorescence behavior of this compound (Fig. 5) [25]. The crystals of 5 adopted a cavernous channel which provided available space to withstand external pressures. The piezochromic properties of the crystal were investigated using anisotropic grinding and isotropic compression. Upon grinding, the wavelength of the PL spectrum was redshifted, displaying yellow emission. The color of the crystal underwent a remarkable variation from the initial green to red when high pressure (9.21 GPa) was applied, accompanied with the great fluorescence red shift. When the pressure was up to 9.21 GPa, the emission wavelength changed to 684 nm which was redshifted by about 155 nm compared with that of the original sample. After the pressure was released, both color and fluorescence of the crystal completely recovered to the original state. The emission peak wavelengths exhibited a linear relationship with the applied pressure in both pressurizing and depressurizing cycles. The in situ Raman spectra of 5 proved that the present piezochromic behavior is induced by the enhanced intermolecular interactions rather than by a phase transition during the pressurizing-depressurizing process. Thus, the DAC technique can induce significant luminescence shift of organic crystals without the destruction of crystal phase.
|
Download:
|
| Figure 5. (a) PL spectra of a CzCNDSB crystal during the pressurizing process, (b) PL spectra of the CzCNDSB crystal during the depressurizing process, (c) plot of the relative emission wavelength and intensity vs. various pressures from 1 atm to 9.21 GPa, (d) images of CzCNDSB crystal in DAC during a pressurizing and depressurizing cycle; excited by a laser (λex = 355 nm). Adapted from Ref. [25] with permission from The Royal Society of Chemistry. | |
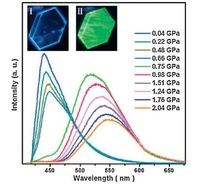
On the contrast, Ma and coworkers show us an example that underwent a phase transition by applying the high pressure on a luminescent organic crystal [26]. They obtained two polymorphs based on a cyano-substituted distyrylbenzene compound 6: a quadrilateral-like crystal with blue fluorescence B-phase (λmax= 445 nm), and a striπ-like crystal with green fluorescence G-phase (λmax= 503nm). The crystal structures of both polymorphs have been determined by X-ray diffraction analysis which provides accurate information about the molecular conformation, intermolecular interactions as well as supramolecular arrangements. When B-phase is heated to its critical temperature (185 ℃) or pressurized to its critical pressure (0.75 GPa), a transition from the B-phase to the G-phase occurred (Fig. 6). In the initial phase, the molecular configuration continuously became planar from a high twisting shape upon external stimuli, on the basis of the gradual change of the fluorescence spectra. Above the critical point, the molecules in the crystal reassembled into a new thermodynamic equilibrium state, corresponding to a sudden change in the emission color. These investigations not only provide a new insight into the organic crystal phase transition; on the other hand, the new phenomenon of piezochromic behavior based on Ma’s result may contribute to process innovation in raster and optical information storage devices. Generally, the phase transformation of organic crystals is induced by either thermally heating or photo irradiation. Ma’s work suggested that hydrostatic pressure produced by the DAC could also trigger crystal-to-crystal transformation.
|
Download:
|
| Figure 6. Photoluminescence (PL) spectra of CN-DSB crystal under increasing pressure starting from the B-phase. Inset images show the CN-DSB crystal in a diamond anvil cell (DAC) excited by laser (λ = 405 nm); (I) at ambient pressure, (II) at 0.75 GPa. (The size of the crystal tested for PL spectra s approximately 100×150 mm2).Adapted from Ref.[26] with permission from The Royal Society of Chemistry. | |
Although compounds 5 and 6 hold the same π-conjugated skeleton, their crystals display totally different pressure-induced piezochromic behaviors. Thus, the molecular microenvironment of luminescent organic crystals is very important for the mechan- oluminescence. In the sense, the desired piezochromic properties can be achieved by not only molecular design but also crystal engineering.
3.3. Triphenylamine crystal and derivativesTriphenylamine (TPA) is a typical non-planar molecule which has been widely employed as functional group in constructing organic materials for fabricating high-performance optoelectronic devices such as organic light-emitting materials studies [27, 28].
Xu and coworkers gave a research on comparison of shearing force and hydrostatic pressure on molecular structures of crystalline TPA by fluorescence and Raman spectroscopies [29] . Upon mechanical grinding by a pestle, the crystal sample changes into an amorphous phase and shows a minor wavelength shift (from 443 to 435 nm) accompanied by greatly reduction of fluorescence intensity. The shearing force destroyed the regular molecular packing and resulted in a phase transformation of the sample from crystal phase to amorphous one. Therefore, the intramolecular vibrational rotations are more flexible, which significantly weakens the fluorescence intensity. Compared with grinding, DAC is used in higher pressures study. When the pressure is less than 0.1 GPa, the emission wavelength shows a small blue shift, which is in accordance with the spectral change under grinding. As the external pressure increase, the fluorescence intensity of TPA gradually increased, accompanied by a slight red shift of the emission band by several nanometers. When the pressure reaches 1.9 GPa, the maximum pressure of the experiment in Xu’s group, the fluorescence intensity significantly increase (about two times) compared with that at atmospheric pressure. The in-situ high-pressure Raman experiments indicate that the TPA molecule tends to flatten. Correspondingly, the steric hindrance between the phenyl rings decreased when external pressure was applied, which makes the molecular structure closepacking. Due to the effective restriction of molecular rotations, the non-radiation transition of TPA solids weakens and the fluorescence intensity increases. Xu’s work demonstrates that the DAC equipment can provide insight into the piezochromism of organic crystals under not only high pressure but also low pressure conditions.
Zhang and Ma et al recently have designed a typical donor- acceptor type (D-A) molecule 7 containing a twisted diphenyla- crylonitrile unit and a triphenylamine group. The multicolored switching of fluorescence (Fig. 7) of this material in responsive to mechanical grinding and isotropic compression were reported [30] .Thecrystallinepowderofcompound 7 (Gform)emittedgreen fluorescence peaked at 507 nm. Upon gentle grinding by a spatula, the fluorescence color of G-form crystal changed immediately to yellow (Y form). The resulted solid converted to an orange sample (O form), which emitted at 608 nm, with further mechanical action. Interestingly, after extensive grinding, the powder ultimately showed a red emission at 618nm (R form) which was significantly redshifted by about 111 nm compared with that of G- form crystal. G form crystals also showed noticeable emission color transitions from initial green to yellow and finally to red during the compression process. Notably, in the high pressure experiments, the emission at 550 nm under a pressure of 1.49 GPa was similar to that of the Y form powders. When the pressure reaches a high level of 6.09 GPa, the crystal emitted faint luminescence at 608 nm, which was blueshifted by 20 nm relative to that of the R form powder. The multicolored transitions were found to be relative to two different kinds of excited state alteration. Green to yellow fluorescence conversion (G form to Y form) is due to the LE-state to the CT-state transformation of a small amount of the original powder. The change of the solid into red is originated from the further conversion to the main existence of the CT-state. The tremendous shift of fluorescence wavelength in a wide range was due to the excited state transformation, which was accompanied by a change of intramolecular conformation and intermolecular packing modes, as demonstrated by XRD, lifetime and Raman spectra analysis. The successive regulation of pressure is one of the advantages ofDAC based on which the detailed emission variations of crystals can be fully recorded.

|
Download:
|
| Figure 7. Digital images of pCN-TPA in different phases recorded under UV light (l = 365 nm): (A) slight (gentle) grinding (Y-form), (B) pristine powder (G-form), (C) extended (fully) grinding (R-form). The letters of "HZU" were written on the "paper" with a spatula: (D) pristine aggregates without force perturbation, (E) full shearing of the aggregates, and (F) slight shearing of the aggregates. (G) Digital images of samples (E) under natural light. (H) Fluorescence images of the crystal under hydrostatic pressure (from compression to decompression). Adapted from Ref. [30] with permission from The John Wiley and Sons. | |
As one of the important structural units for functional organic materials, TPA is also active in constructing piezochromic organic crystals. Under high-pressure conditions, TPA units may undergo conformational change which affects the molecular rotation and/ or electronic structure of the entire molecule and hence results in mechanoluminescence.
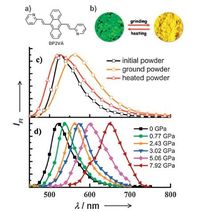
3.4. Anthracene derivativesRecently, Tian and coworkers reported a series of divinylan- thracene derivatives with fascinating optical properties [31, 32]. In 2012, they reported an anthracence-containing compound 8 with piezochromic characteristics [33]. Both grinding and exertion of external pressure on the powder led to a change in its photoluminescence color from green to red (Fig. 8). At ambient conditions, its powder exhibited a strong green emission peaked at 528 nm. After being ground, the powder showed a strong redshift of fluorescence and exhibited a yellow emission centered at 561 nm. Under high pressure, the fluorescence spectrum of the powder showed a gradual redshift. When the applied pressure increased to 7.92 GPa, the fluorescence color of the powder sample changed from green (528 nm) to red (652 nm). Based on molecular packing structure analysis of polymorph-dependent crystals, they proposed the reasonable mechanism about the piezochromic properties of 8. As the pressure increased, external pressure impelled the formation of molecular aggregation state, which transformed from J-type aggregation to H-type aggregation, and further to the much tighter face-to-face stack like dimmers. At the same time, the intermolecular π-π interaction strengthened gradually which induced PL spectrum shift from green (without π-π interaction) to orange (with weak π-π interaction) and finally to red emission (with strong π-π interaction). Therefore, changeable molecular aggregation state under grinding or pressure can induce the tunable fluorescence based on which the mechanochromic effect generated in the powder of 8. Similar pressure induced mechanochromic property was observed by the same group based on an analogues compound 9 [34]. The mechanochromic behavior of this compound originates from the changes in their aggregation state under high external pressure.
|
Download:
|
| Figure 8. (a) Molecular structure of BP2VA. (b) Photographs of the ground powder and the heated powder under UV light (365 nm). (c) PL spectra of the initial, ground, and heated powders. (d) PL spectrum of BP2VA powder under external pressure. FI = fluorescence intensity. Adapted from Ref. [33] with permission from The John Wiley and Sons. | |
3.5. Tetraphenylethylene derivatives
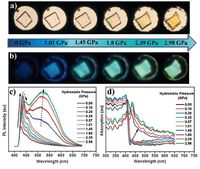
Some solution-state not emissive organic molecules are found to produce brightly fluorescent solids. This phenomenon was defined as aggregation-induced emission (AIE) in 2001 by Tang and coworkers [35]. Tetraphenylethylene (TPE) derivatives with AIE feature were first reported by Tang et al. [36-38]. Very recently, Tian and coworkers have presented a novel molecule 10 that exhibits the remarkable turn-on and color-tuned luminescence under mechanical grinding or hydrostatic compression [39]. The almost orthogonal conformation between TPE unit and AD substituent completely separated the electronic distribution, which inhibited the ICT process and hence lead to the emission from LE state, resulting in the dark-phase of the crystals. The heavily twisted conformation could be planarized simply by the force perturbation when the molecule is under the mechanical stimuli, which resulted in an overlap of the frontier orbitals between donor and acceptor and the formation of ICT state. Thus, the switching of excited state characteristics by the mechanical stimuli induced the change of luminescence from the non-emissive dark-phase to the bright-phase (Fig. 9). This report shows a rare example of high contrast ratio mechanochromic luminescent materials with the concept of a mechanical switching of the excited state. In addition, Zou and coworkers reported the distinctive luminescence response of TPE crystal to extreme compression and provided deep insight into the mechanism of AIEE-active materials [40]. The switching of excited state characteristics of organic luminescent compounds was generally studied in solutions with different polarity. The DAC equipment pave a way to elucidate the excited state transformation of organic solids based on the pressure-induced molecular conformation change.
|
Download:
|
| Figure 9. (a and b) Visible and fluorescence images of a single crystal of AD-TPE under different hydrostatic pressures. (c and d) Corresponding fluorescence and absorption spectra. Adapted from Ref. [39] with permission from The John Wiley and Sons. | |
Xu and coworkers studied the mechanochromic characteristic of 11 using fluorescence and Raman spectroscopies under the hydrostatic pressure via a DAC [41]. Under the hydrostatic pressure, the geometry of 11 started to distort through the change of the dihedral angle between the benzene ring and the planar ethylene core. As a result, the molecules became to be close- packed. And the π-π stacking interaction among 11 molecules was enhanced, resulting in the quenching of its fluorescence (Fig. 10). The C-H ⋅⋅⋅ O hydrogen bonds between molecules were strengthened with increasing pressure, which together with the enhanced π-π interaction is responsible for the obvious red shift of the fluorescence band. Raman peaks under the hydrostatic pressure and the theoretical calculation both prove that the distortion of the torsion angle leads to enhanced intermolecular interactions and facilitates C-H- - O hydrogen bond interactions. This study provides experimental evidence that the intramolecular interaction is responsible for piezofluorochromic aggregation-induced emission (PAIE). The authors suggested that the proposed mechanism can be extended to other piezochormic materials with AIE character and weak intermolecular interaction.

|
Download:
|
| Figure 10. Fluorescence spectra of TMOE (A) and TPE (B) under different pressures. The numbers indicate the pressure in units of gigapascals. Excitation wavelength was 365 nm. (Inset) Photographs of partial TMOE and TPE crystals before and after hydrostatic pressure was performed,taken by a camera fixed on the fluorescence microscope. Adapted with permission from Ref. [41]. Copyright 2015,American Chemical Society. | |
3.6. Other organic materials
In 2013, Yamaguchi and coworkers reported the distinct luminescent responses of a tetrathiazolylthiophene fluorophore 12 to anisotropic grinding and isotropic compression [42]. The crystalline sample of this compound displayed yellow emission peaked at 556 nm which was significantly redshifted compared with that of solution sample or doped thin film. The excimer formation in the three-dimensional hydrogen-bond network was responsible for the yellow emission of the crystalline powder. Mechanical grinding of the crystals leads to an increase of the disordered phase in which the excimer formation was suppressed (Fig. 11). As a result,the ground sample showed blueshifted fluorescence with emission maximum at 490 nm. In sharp contrast,the yellow excimer emission of the crystals could redshift to orange fluorescence centered at 609 nm when high pressure of about 3.2 GPa was applied. The single-crystal XRD analysis at high pressure unambiguously determined the structure changes during compressing. The compression-induced closer arrangement of the face-to-face dimer of the fluorophore is the origin of the more redshifted excimer emission. The reversible structural deformation of the hydrogen-bonding interaction based 3D network upon compression/decompression was confirmed by both theoretical and experimental investigations. Although the powders showed yellow fluorescence,this fluorophore exhibits deep blue fluorescence at 449 nm in the PMMA doped film. In this sense,the multicolor luminescence covering a wide visible region was achieved by this research group based on a single organic compound only by changing the solid state and the types of the mechanical forces. This example shows another advantage of DAC that the accurate crystal structure of organic materials can be determined under high-pressure conditions.

|
Download:
|
| Figure 11. Tetrathiazolylthiophene and its yellow-emissive crystals (left) and distinct luminescent responses to mechanical grinding,producing a green-emissive powder,and to hydrostatic pressure,affording an orange-emissive crystal (right). Adapted with permission from Ref. [42]. Copyright 2013,American Chemical Society. | |
Zhang and coworkers prepared a polycrystalline fluorophore 13 which forms three organic polymorphs (BCrys, SCrys and YCrys) with blue, sky-blue, and yellow fluorescence [43]. On the basis of single-crystal structural and photophysical analysis, they found that the different fluorescence efficiencies of the polymorphs could be attributed to the conformational change rather than the intermolecular interaction. The intramolecular motions in the crystalline state quenched the fluorescence, which has been confirmed by in situ steady-state PL experiments. The PL spectra indicated that changes in the molecular aggregate state of BCrys and SCrys upon grinding or under high pressure resulted in the altering of molecular conformation, and thus induced mechan- ochromic fluorescence.
4. ConclusionsHigh pressure provides an ideal pattern to study the mechan- ochromic system. Isotropic pressure that generated by diamond anvil cell are quite different than the traditional methods and may directly have effect on the molecular properties. The above reports we summarized including diverse kind of materials, such as organoboron compounds, TPE and TPA derivatives, AIE materials etc. These materials have outstanding mechanoluminescence under high pressure and most of their intra- and intermolecular interactions can be changed reversibly and reproducibly. On the basis of these typical examples, the general effects of high pressure on luminescent properties of organic solids can be ruled out as follows: the isotropic compression produced by hydrostatic pressure may induce molecular planarizartion and/or tightly packed structure, both of which generally cause red shift of emission. In this sense, molecules with conformation flexibility as well as loose packing structures in solid forms are good candidates for realizing high-pressure induced mechanoluminescecne. The applied of DAC measurement in studying mechanochromic materials give us in-depth insight to the relationship between luminescence and molecular structure. These reports will expand the range of mechano-responsive luminescent materials.
Acknowledgment This work was supported by the National Natural Science Foundation of China (No. 91333201).| [1] | Y.Q. Dong, J.W.Y. Lam, B.Z. Tang. Mechanochromic luminescence of aggregationinduced emission Luminogens. J. Phys. Chem. Lett. 6 (2015) 3429–3436. DOI:10.1021/acs.jpclett.5b01090 |
| [2] | X.Y. Mu, D. Liu, X. Cheng, et al. AuI AuI interaction induced semiconducting microwires with photo- and vapor-responsive properties. Org. Electron. 13 (2012) 457–463. DOI:10.1016/j.orgel.2011.11.013 |
| [3] | J. Feng, K.J. Tian, D.H. Hu, et al. A triarylboron-based fluorescent thermometer: sensitive over a wide temperature range, Angew. Angew. Chem. Int. Ed. 50 (2011) 8072–8076. DOI:10.1002/anie.v50.35 |
| [4] | T. Sato, M. Higuchi. A vapoluminescent Eu-based metallo-supramolecular polymer. Chem. Commun. 48 (2012) 4947–4949. DOI:10.1039/c2cc30972f |
| [5] | W.R. Browne, M.M. Pollard, B. de Lange, A. Meetsma, B.L. Feringa. Reversible threestate switching of luminescence: a new twist to electro- and photochromic behavior. J. Am. Chem. Soc. 128 (2006) 12412–12413. DOI:10.1021/ja064423y |
| [6] | X. Cheng, K. Wang, S. Huang, et al. Organic crystals with near-infrared amplified spontaneous emissions based on 2'-hydroxychalcone derivatives: subtle structure modification but great property change. Angew. Chem. Int. Ed. 54 (2015) 8369–8373. DOI:10.1002/anie.201503914 |
| [7] | K. Wang, H.Y. Zhang, S.Y. Chen, et al. Organic polymorphs: one-compound-based crystals with molecular-conformation- and packing-dependent luminescent properties. Adv. Mater. 26 (2014) 6168–6173. DOI:10.1002/adma.201401114 |
| [8] | X. Cheng, D. Li, Z.Y. Zhang, H.Y. Zhang, Y. Wang. Organoboron compounds with morphology-dependent NIR emissions and dual-channel fluorescent ON/OFF switching. Org. Lett. 16 (2014) 880–883. DOI:10.1021/ol403639n |
| [9] | B.L. Tang, H.P. Liu, F. Li, Y. Wang, H.Y. Zhang. Single-benzene solid emitters with lasing properties based on aggregation-induced emissions. Chem. Commun. 52 (2016) 6577–6580. DOI:10.1039/C6CC02616H |
| [10] | X. Cheng, Y.F. Zhang, S.H. Han, et al. Multicolor amplified spontaneous emissions based on organic polymorphs that undergo excited-state intramolecular proton transfer. Chem. Eur. J. 22 (2016) 4899–4903. DOI:10.1002/chem.v22.14 |
| [11] | J.A. Xu, H.K. Mao, P.M. Bell. High-pressure ruby and diamond fluorescence: observations at 0.21 to 0.55 terapascal. Science 32 (1986) 1404–1406. |
| [12] | L. Dubrovinsky, N. Dubrovinskaia, V.B. Prakapenka, A.M. Abakumov. Implementation of micro-ball nanodiamond anvils for high-pressure studies above 6 Mbar. Nat. Commun. 3 (2012) 1163. DOI:10.1038/ncomms2160 |
| [13] | K. Wang, S.R. Li, X. Tan, et al. High pressure supramolecular chemistry. Chin. Sci. Bull. 59 (2014) 5258–5268. DOI:10.1007/s11434-014-0615-9 |
| [14] | R. Lee, J.A.K. Howard, M.R. Probert, J.W. Steed. Structure of organic solids at low temperature and high pressure. Chem. Soc. Rev. 43 (2014) 4300–4311. DOI:10.1039/c4cs00046c |
| [15] | D. Li, H.Y. Zhang, Y. Wang. Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs). Chem. Soc. Rev. 42 (2013) 8416–8433. DOI:10.1039/c3cs60170f |
| [16] | S.N. Wang. Luminescence and electroluminescence of Al(III), B(III), Be(II) and Zn(II) complexes with nitrogen donors. Coord. Chem. Rev. 215 (2001) 79–98. DOI:10.1016/S0010-8545(00)00403-3 |
| [17] | L.S. Hung, C.H. Chen. Recent progress of molecular organic electroluminescent materials and devices. Mater. Sci. Eng. R 39 (2002) 143–222. DOI:10.1016/S0927-796X(02)00093-1 |
| [18] | L. Wang, K. Wang, B. Zou, et al. Luminescent chromism of boron diketonate crystals: distinct responses to different stresses. Adv. Mater. 27 (2015) 2918–2922. DOI:10.1002/adma.v27.18 |
| [19] | L. Wang, K. Wang, H.Y. Zhang, et al. The facile realization of RGB luminescence based on one yellow emissive four-coordinate organoboron material. Chem. Commun. 51 (2015) 7701–7704. DOI:10.1039/C5CC01113B |
| [20] | X.Q. Wang, Q.S. Liu, H. Yan, et al. Piezochromic luminescence behaviors of two new benzothiazole-enamido boron difluoride complexes: intra- and inter-molecular effects induced by hydrostatic compression. Chem. Commun. 51 (2015) 7497–7500. DOI:10.1039/C5CC01902H |
| [21] | H. Wang, F. Li, B.R. Gao, et al. Doped organic crystals with high efficiency, colortunable emission toward laser application. Cryst. Growth Des. 9 (2009) 4945–4950. DOI:10.1021/cg9007125 |
| [22] | S.J. Yoon, S.Y. Park. Polymorphic and mechanochromic luminescence modulation in the highly emissive dicyanodistyrylbenzene crystal: secondary bonding interaction in molecular stacking assembly. J. Mater. Chem. 21 (2011) 8338–8346. DOI:10.1039/c0jm03711g |
| [23] | M.S. Kwon, J. Gierschner, S.J. Yoon, S.Y. Park. Unique piezochromic fluorescence behavior of dicyanodistyrylbenzene based donor-acceptor-donor triad: mechanically controlled photo-induced electron transfer (eT) in molecular assemblies. Adv. Mater. 24 (2012) 5487–5492. DOI:10.1002/adma.v24.40 |
| [24] | S.J. Yoon, J.W. Chung, J. Gierschner, et al. Multistimuli two-color luminescence switching via different slip-stacking of highly fluorescent molecular sheets. J. Am. Chem. Soc. 132 (2010) 13675–13683. DOI:10.1021/ja1044665 |
| [25] | C.F. Feng, K. Wang, Y.X. Xu, et al. Unique piezochromic fluorescence behavior of organic crystal of carbazole-substituted CNDSB. Chem. Commun. 52 (2016) 3836–3839. DOI:10.1039/C5CC09152G |
| [26] | Y.X. Xu, K. Wang, Y.J. Zhang, et al. Fluorescence mutation and structural evolution of a p-conjugated molecular crystal during phase transition. J. Mater. Chem. C 4 (2016) 1257–1262. DOI:10.1039/C5TC03745J |
| [27] | H.J. Lee, J. Sohn, J. Hwang, et al. Triphenylamine-cored bifunctional organic molecules for two-photon absorption and photorefraction. Chem. Mater. 16 (2004) 456–465. DOI:10.1021/cm0343756 |
| [28] | Z.Y. Ge, T. Hayakawa, S. Ando, et al. Spin-coated highly efficient phosphorescent organic light-emitting diodes based on bipolar triphenylamine-benzimidazole derivatives. Adv. Funct. Mater. 18 (2008) 584–590. DOI:10.1002/(ISSN)1616-3028 |
| [29] | J.X. Wu, H.L. Wang, S.P. Xu, W.Q. Xu. Comparison of shearing force and hydrostatic pressure on molecular structures of triphenylamine by fluorescence and Raman spectroscopies. J. Phys. Chem. A 119 (2015) 1303–1308. DOI:10.1021/jp511380a |
| [30] | Y.J. Zhang, K. Wang, G.L. Zhuang, et al. Multicolored-fluorescence switching of ICT-type organic solids with clear color difference: mechanically controlled excited state. Chem. Eur. J. 21 (2015) 2474–2479. DOI:10.1002/chem.v21.6 |
| [31] | J.B. Zhang, J.L. Chen, B. Xu, et al. Remarkable fluorescence change based on the protonation-deprotonation control in organic crystals. Chem. Commun. 49 (2013) 3878–3880. DOI:10.1039/c3cc41171k |
| [32] | Y.J. Dong, B. Xu, J.B. Zhang, et al. Supramolecular interactions induced fluorescent organic nanowires with high quantum yield based on 9,10-distyrylanthracene. CrystEngComm 14 (2012) 6593–6598. DOI:10.1039/c2ce25276g |
| [33] | Y.J. Dong, B. Xu, J.B. Zhang, et al. Piezochromic luminescence based on the molecular aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl) anthracene. Angew. Chem. Int. Ed. 51 (2012) 10782–10785. DOI:10.1002/anie.v51.43 |
| [34] | Y.J. Dong, J.B. Zhang, X. Tan, et al. Multi-stimuli responsive fluorescence switching: the reversible piezochromism and protonation effect of a divinylanthracene derivative. J. Mater. Chem. C 1 (2013) 7554–7559. DOI:10.1039/c3tc31553c |
| [35] | J.D. Luo, Z.L. Xie, J.W.Y. Lam, et al., Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun. (2001) 1740-1741. |
| [36] | X.L. Luo, W.J. Zhao, J.Q. Shi, et al. Reversible switching emissions of tetraphenylethene derivatives among multiple colors with solvent vapor, mechanical, and thermal stimuli. J. Phys. Chem. C 116 (2012) 21967–21972. DOI:10.1021/jp306908u |
| [37] | N. Zhao, Z.Y. Yang, J.W.Y. Lam, et al. Benzothiazolium-functionalized tetraphenylethene: an AIE luminogen with tunable solid-state emission. Chem. Commun. 48 (2012) 8637–8639. DOI:10.1039/c2cc33780k |
| [38] | J. Wang, J. Mei, R.R. Hu, et al. Click synthesis, aggregation-induced emission, E/Z isomerization,self-organization,andmultiplechromismsofpurestereoisomersof a tetraphenylethene-cored luminogen. J. Am. Chem. Soc. 134 (2012) 9956–9966. DOI:10.1021/ja208883h |
| [39] | Q.K. Qi, J.Y. Qian, X. Tan, et al. Remarkable turn-on and color-tuned piezochromic luminescence: mechanically switching intramolecular charge transfer in molecular crystals. Adv. Funct. Mater. 25 (2015) 4005–4010. DOI:10.1002/adfm.v25.26 |
| [40] | H.S. Yuan, K. Wang, K. Yang, B.B. Liu, B. Zou. Luminescence properties of compressed tetraphenylethene: the role of intermolecular interactions. J. Phys. Chem. Lett. 5 (2014) 2968–2973. DOI:10.1021/jz501371k |
| [41] | J.X. Wu, J. Tang, H.L. Wang, et al. Reversible piezofluorochromic property and intrinsic structure changes of tetra(4-methoxyphenyl)ethylene under high pressure. J. Phys. Chem. A 119 (2015) 9218–9224. DOI:10.1021/acs.jpca.5b02362 |
| [42] | K. Nagura, S. Saito, H. Yusa, et al. Distinct responses to mechanical grinding and hydrostatic pressure in luminescent chromism of tetrathiazolylthiophene. J. Am. Chem. Soc. 135 (2013) 10322–10325. DOI:10.1021/ja4055228 |
| [43] | Y.J. Zhang, Q.B. Song, K. Wang, et al. Polymorphic crystals and their luminescence switching of triphenylacrylonitrile derivatives upon solvent vapour, mechanical, and thermal stimuli. J. Mater. Chem. C 3 (2015) 3049–3054. DOI:10.1039/C4TC02826K |
 2016, Vol. 27
2016, Vol. 27 


